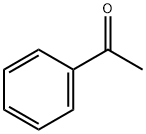
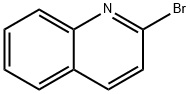
1-phenyl-2-(quinolin-2-yl)ethan-1-one synthesis
- Product Name:1-phenyl-2-(quinolin-2-yl)ethan-1-one
- CAS Number:1531-38-0
- Molecular formula:C17H13NO
- Molecular Weight:247.29
Yield:1531-38-0 56.7%
Reaction Conditions:
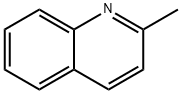
Stage #1: 2-methylquinolinewith n-butyllithium in tetrahydrofuran at 20; for 3 h;Inert atmosphere;
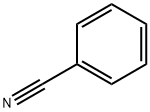
Stage #2: benzonitrile in tetrahydrofuran at 20; for 48 h;Inert atmosphere;
Stage #3: with sulfuric acid pH=1; for 24 h;Cooling with ice;Inert atmosphere;
Steps:
1 Example 1: Preparation of ligand 2-(2-quinolyl)acetophenone
Under a nitrogen atmosphere, 2-methyl-quinoline (29.14mmol, 3.94ml) added to the reaction flask, followed by addition of 100mL of tetrahydrofuran was stirred sufficiently to dissolve.Under ice-cooling, was added slowly dropwise twice equivalents of n-butyllithium (58.28mmol, 23.13mL), the color of the solution changes to dark red, after half an hour the ice bath was restored to room temperature and stirring was continued 3H; Under ice-cooling, taking benzonitrile (29.14mmol, 2.98ml) was slowly added dropwise to the reaction solution, the solution became cloudy white powder which have occurred.Recovery After half an hour the reaction to room temperature, the reaction was stirred for 2 days.Under ice-cooling, a 60% concentrated sulfuric acid was added to the reaction solution to pH = 1, stirring was continued for 1 day.Saturated KOH and the reaction solution to pH = 7 ~ 8, and extracted three times with dichloromethane to give a dark red clear solution, adding an appropriate amount of anhydrous magnesium sulfate for one day spin dry solvent, a dark red knot blocks of matter.Was added n-hexane: dichloromethane = 1: 1 and recrystallized to give an orange-red solid was precipitated, 4.078g, yield 56.7%.1HNMR (400MHz, of DMSO) [delta] = 6.26 (S, IH), 7.05 (D, J = 9.1Hz, IH), 7.25 (T, J = 7.3Hz, IH), 7.36-7.42 (m, 3H), 7.53 (t, J = 7.6Hz, 1H ), 7.60 (d, J = 8.2Hz, 1H), 7.64 (d, J = 7.8Hz, 1H), 7.83-7.92 (m, 3H), 15.57 (s, 1H).
References:
CN104031075,2016,B Location in patent:Paragraph 0017; 0018

70437-00-2
0 suppliers
inquiry

1531-38-0
11 suppliers
inquiry