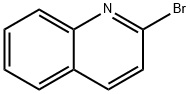
2-Bromoquinoline synthesis
- Product Name:2-Bromoquinoline
- CAS Number:2005-43-8
- Molecular formula:C9H6BrN
- Molecular Weight:208.05
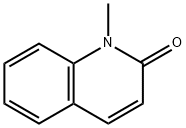
606-43-9

2005-43-8
The general procedure for the synthesis of 2-bromoquinoline from 1-methyl-2-quinolone was as follows: Triphenylphosphine (1186 mg, 4.52 mmol) and dibromoisocyanuric acid (652 mg, 2.27 mmol) were sequentially added to a 50 mL round bottom flask fitted with a magnetic stirrer and a balloon (for pressure equilibrium) under nitrogen protection. The mixture was heated to 115 °C, at which point the dibromoisocyanuric acid reacted vigorously, and the reaction was continued to maintain this temperature for 10 min. Subsequently, 1-methylquinolin-2(1H)-one (239 mg, 1.50 mmol) was added to the reaction system and the temperature of the reaction was raised to 160-170°C for 16 hours. After completion of the reaction, the reaction mixture was dissolved in dichloromethane and alkalized with triethylamine. Purification was carried out by silica gel column chromatography with hexane-ethyl acetate (6:1, v/v) as eluent to finally obtain 2-bromoquinoline (244 mg, 78% yield). 2-bromoquinoline was a yellow oil, and its 1H-NMR (CDCl3) data were as follows: δ 7.51 (1H, d, J = 8.4 Hz), 7.51-8.20 (4H, m), 7.98 ( 1H, d, J = 8.4Hz).
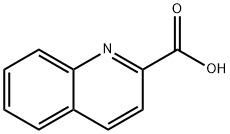
93-10-7
330 suppliers
$6.00/5g

2005-43-8
213 suppliers
$6.00/250mg
Yield:2005-43-8 75%
Reaction Conditions:
with tert-butylhypochlorite;sodium carbonate;sodium bromide at 60; for 20 h;Schlenk technique;
Steps:
10 Example 10: Synthesis of 2-bromoquinoline (2j)
Weighing quinoline-2-carboxylic acid (51.9 mg, 0.3 mmol),Sodium carbonate (64.0 mg, 0.6 mmol), NaBr (17.6 mg, 0.3 mmol), tert-butyl hypochlorite (32 μL, 0.3 mmol) into a 25 mL of Schlenk reaction bottle, Then CH2Br2 (2 mL) was added and placed in a 60 °C oil bath for 20 h. After completion of the reaction, the solvent was removed under reduced pressure and eluted with petroleum ether / ethyl acetate.The solvent was separated on a silica gel column, he yield of 2-bromoquinoline was 75%.
References:
Dalian University of Technology;Feng Xiujuan;Zhang Xitao;Zhang Haixia;Bao Ming CN108586334, 2018, A Location in patent:Paragraph 0072-00374

606-43-9
76 suppliers
inquiry

2005-43-8
213 suppliers
$6.00/250mg
1613-37-2
0 suppliers
$53.00/5G

2005-43-8
213 suppliers
$6.00/250mg
3964-04-3
191 suppliers
$30.00/5g

74857-04-8
0 suppliers
inquiry

2005-43-8
213 suppliers
$6.00/250mg

1613-37-2
0 suppliers
$53.00/5G

2005-43-8
213 suppliers
$6.00/250mg