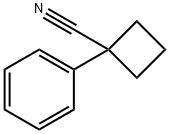
1-Phenylcyclobutanecarbonitrile synthesis
- Product Name:1-Phenylcyclobutanecarbonitrile
- CAS Number:14377-68-5
- Molecular formula:C11H11N
- Molecular Weight:157.21
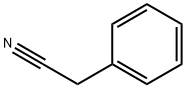
140-29-4

109-64-8

14377-68-5
The general procedure for the synthesis of 1-phenylcyclobutanecarbonitrile from phenylacetonitrile and 1,3-dibromopropane was as follows: powdered potassium hydroxide (536 g, 9.56 mol, 5.6 eq.) was suspended in a solvent mixture of toluene (1.54 L) and water (154 mL) and heated to 45 °C. Subsequently, tetrabutylammonium bromide (28 g, 0.85 mol, 0.05 eq.) and 1,3-dibromopropane (379 g, 1.88 mol, 1.1 eq.) were added followed by the slow dropwise addition of a toluene (500 mL) solution of phenylacetonitrile (200 g, 1.7 mol, 1.0 eq.) over a period of 42 min. During the dropwise addition, the reaction temperature was raised to 95 °C and the mixture was heated to reflux after the dropwise addition was completed. The resulting pink slurry was stirred at reflux temperature for 1 h. The completion of the reaction was confirmed by HPLC analysis. The reaction mixture was cooled to 20-25 °C and filtered through a Celite pad. The solid residue was washed with toluene (1.0 L), and the combined filtrates were washed sequentially with water (2 × 300 mL) and brine (150 mL), dried over anhydrous magnesium sulfate and filtered, and concentrated to give the crude product 1-phenylcyclobutanecarbonitrile (263 g) as an orange oil. The crude product was purified by vacuum distillation (boiling point 105 °C/750 mT) to give 1-phenylcyclobutanecarbonitrile in colorless liquid form [140 g, yield 52%, purity 97.7% (AUC)]. The major impurity was identified as B2 (2.3% AUC) (see Example 9, Synthesis of B section for details).

140-29-4
0 suppliers
$18.00/25mL

109-64-8
506 suppliers
$10.00/5g

14377-68-5
117 suppliers
$14.00/1g
Yield: 87.6%
Reaction Conditions:
Stage #1:phenylacetonitrile with sodium hydride in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2:1,3-dibromo-propane in N,N-dimethyl-formamide at 20; for 4 h;
Steps:
48 Synthesis of 1-phenylcyclobutane-1-nitrile (48A)
Dissolve NaH (1.02g, 42.68mmol) in DMF (15mL), slowly add benzylacetonitrile (2.00g, 17.07mmol) dropwise at 0, stir for 0.5h, add 1,3-dibromopropane (3.45g, 17.07mmol), react at room temperature for 4h, add 30mL ethyl acetate and 100mL water, separate the organic layer, extract the aqueous layer with ethyl acetate (30mL×3), combine the organic layers, wash with saturated brine, and dry with anhydrous magnesium sulfate. Suction filtration, concentration under reduced pressure, separation and purification by column chromatography to obtain 2.35 g of colorless oil, yield 87.6%,
References:
CN113061098, 2021, A Location in patent:Paragraph 0390-0393

140-29-4
0 suppliers
$18.00/25mL

14377-68-5
117 suppliers
$14.00/1g

140-29-4
0 suppliers
$18.00/25mL

142-28-9
282 suppliers
$10.00/1g

14377-68-5
117 suppliers
$14.00/1g