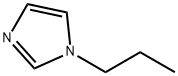
1-Propyl-1H-imidazole synthesis
- Product Name:1-Propyl-1H-imidazole
- CAS Number:35203-44-2
- Molecular formula:C6H10N2
- Molecular Weight:110.16

288-32-4

106-94-5

35203-44-2
Synthesis of 1-propyl-1H-imidazole: Sodium hydride (55%, 0.966 g, 22.1 mmol) was slowly added to a solution of tetrahydrofuran (50.0 mL) of imidazole (1.37 g, 20.1 mmol) at room temperature. The reaction mixture was stirred at room temperature for 1 hour. Subsequently, 1-bromopropane (5.48 mL, 60.3 mmol) was added dropwise at the same temperature and the reaction solution was continued to be stirred for 16 hours. Upon completion of the reaction, the reaction mixture was filtered through a diatomaceous earth pad and the filter cake was washed with tetrahydrofuran. The filtrate and washings were combined and concentrated under reduced pressure to remove the solvent. The resulting crude product was purified by fast column chromatography (silica gel as stationary phase and chloroform/methanol as eluent) to afford 1-propyl-1H-imidazole (2.07 g, 18.8 mmol, 93% yield) as a colorless oil.1H NMR (400 MHz, CDCl3) δ: 0.93 (3H, t, J = 7.2 Hz), 1.81 (2H, td, J = 7.2, 14.4 Hz), 3.90 (2H, t, J = 7.2 Hz), 6.91 (1H, s), 7.06 (1H, s), 7.46 (1H, s).

288-32-4
1039 suppliers
$5.00/25g

106-94-5
583 suppliers
$10.00/5 mL

35203-44-2
252 suppliers
$6.00/5g
Yield: 100%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;mineral oil at 0 - 20;Inert atmosphere;
References:
Serpell, Christopher J.;Cookson, James;Thompson, Amber L.;Brown, Christopher M.;Beer, Paul D. [Dalton Transactions,2013,vol. 42,# 5,p. 1385 - 1393]

288-32-4
1039 suppliers
$5.00/25g
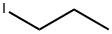
107-08-4
260 suppliers
$14.00/5g

35203-44-2
252 suppliers
$6.00/5g
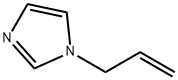
31410-01-2
196 suppliers
$14.00/5g

35203-44-2
252 suppliers
$6.00/5g

106-94-5
583 suppliers
$10.00/5 mL
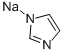
5587-42-8
72 suppliers
$121.00/10g

35203-44-2
252 suppliers
$6.00/5g
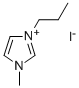
119171-18-5
128 suppliers
$11.00/1g

35203-44-2
252 suppliers
$6.00/5g

74-88-4
357 suppliers
$15.00/10g