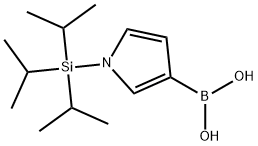
1-(Triisopropylsilyl)pyrrole-3-boronic acid synthesis
- Product Name:1-(Triisopropylsilyl)pyrrole-3-boronic acid
- CAS Number:138900-55-7
- Molecular formula:C13H26BNO2Si
- Molecular Weight:267.25
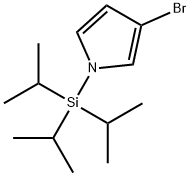
87630-36-2

138900-55-7
Step 92b: Synthesis of 1-(triisopropylsilyl)pyrrole-3-boronic acid (Compound 0602-197) To a stirring solution of 3-bromo-1-(triisopropylmethylsilyl)pyrrole (0601-197, 1 g, 3.31 mmol) in anhydrous THF (20 mL) under nitrogen protection, a THF solution of n-butyllithium (2.5 M, 1.58 mL, 3.96 mmol) was slowly added at -78 °C. Maintaining this temperature, stirring was continued for 30 minutes. Subsequently, trimethyl borate (687 mg, 6.6 mmol) was added dropwise to the reaction mixture. The reaction mixture was gradually warmed up to room temperature and stirring was continued for 1 hour. After completion of the reaction, the mixture was diluted with 200 mL of water and extracted with 200 mL of ethyl acetate. The organic phase was washed sequentially with 100 mL of water (twice) and 100 mL of brine and dried over anhydrous sodium sulfate. After concentration under reduced pressure, the crude product 0602-197 (280 mg, 32% yield) was obtained as an oil, which was used directly in the next step of the reaction without further purification.LCMS detection showed a molecular ion peak of 268 [M + 1]+.

87630-36-2
132 suppliers
$17.00/250mg

138900-55-7
104 suppliers
$47.00/1g
Yield:138900-55-7 280 mg
Reaction Conditions:
Stage #1:3-bromo-1-triisopropylsilanyl-1H-pyrrole with n-butyllithium in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2: with Trimethyl borate in tetrahydrofuran at -78 - 20; for 1 h;
Steps:
92.92b Step 92b: 1-(Triisopropylsilyl)-1H-pyrrol-3-ylboronic acid (Compound 0602-197)
Step 92b: 1-(Triisopropylsilyl)-1H-pyrrol-3-ylboronic acid (Compound 0602-197)[0608]A solution n-BuLi in THF (2.5 M, 1.58 mL, 3.96 mmol) was added to a stirred solution of 0601-197 (1 g, 3.31 mmol) in anhydrous THF (20 mL) at -78° C. in an N2 atmosphere. The resulting mixture was stirred at this temperature for 30 min. To the mixture was added trimethyl borate (687 mg, 6.6 mmol) dropwise. Then the mixture was warmed to room temperature and stirred for additional 1 h. The mixture was diluted with water (200 mL), extracted with ethyl acetate (200 mL). The organic layer was washed with water (2×100 mL) and brine (100 mL), dried over Na2SO4, concentrated to give crude compound 0602-197 (280 mg, 32%) as a oil which was used in next step directly without further purification. LCMS: 268 [M+1]+.
References:
Curis, Inc.;Bao, Rudi;Lai, Chengjung;Qian, Changgeng US2013/102595, 2013, A1 Location in patent:Paragraph 0608

121-43-7
381 suppliers
$13.00/25mL

87630-36-2
132 suppliers
$17.00/250mg

138900-55-7
104 suppliers
$47.00/1g

121-43-7
381 suppliers
$13.00/25mL
![1H-Pyrrole, 3-iodo-1-[tris(1-methylethyl)silyl]-](/CAS/20210111/GIF/117270-91-4.gif)
117270-91-4
3 suppliers
inquiry

138900-55-7
104 suppliers
$47.00/1g
87630-35-1
171 suppliers
$7.00/250mg

138900-55-7
104 suppliers
$47.00/1g
13154-24-0
446 suppliers
$20.00/10g

138900-55-7
104 suppliers
$47.00/1g