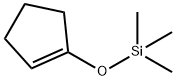
1-(Trimethylsiloxy)cyclopentene synthesis
- Product Name:1-(Trimethylsiloxy)cyclopentene
- CAS Number:19980-43-9
- Molecular formula:C8H16OSi
- Molecular Weight:156.3
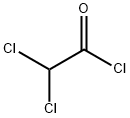
79-36-7
296 suppliers
$14.00/5g

120-92-3
427 suppliers
$11.00/25g

19980-43-9
109 suppliers
$20.00/1 g
Yield:-
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide
Steps:
I Preparation of cycloheptane-1,3-dione:
1-(Trimethylsiloxy)-cyclopentene: A 1 L round bottom flask was charged with cyclopentanone (50.7 g, 0.603 mol) and DMF (250 mL). Triethylamine was added (200 mL, 1.45 mol), followed by dropwise addition of TMSCI (91 mL, 0.72 mol) over 5 min. The solution was then warmed to reflux (90° C.) for 26 h. After cooling to ambient temperature, the mixture was transferred to a separatory funnel, rinsing with 500 mL of hexanes. The solution was washed with water (3 portions of 100 mL each), brine (100 mL), then concentrated to give 110 g of a dark orange oil. 1H nmr analysis showed the desired product, plus 10-15% of triethylamine. This material was used in the next reaction without further purification. 7,7-Dichloro-1-(trimethylsiloxy)-bicyclo[32.0]heptan-6-one: The crude TMS enol ether (2) (0.60 mol) was dissolved in 950 mL of hexanes in a 2 L round bottom flask. Triethylamine (100 mL, 0.72 mol) was added, followed by dichloroacetyl chloride (58 mL, 0.60 mol) as a solution in 450 mL hexanes, dropwise over 2 h. The solution was then stirred at ambient temperature ovemight. The reaction mixture was then filtered through fritted glass, rinsing with several 50 mL portions of hexane. The clear solution was concentrated in vacuo to provide 128 g (80% over 2 steps) of product as a dark brown oil. This material was homogeneous by GC/MS and 1H nmr, save traces of Et3N and DMF, and was used directly in the next reaction.
References:
Pfizer Inc US6262272, 2001, B1
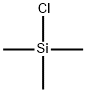
75-77-4
591 suppliers
$5.04/10

120-92-3
427 suppliers
$11.00/25g

19980-43-9
109 suppliers
$20.00/1 g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

19980-43-9
109 suppliers
$20.00/1 g
34880-70-1
52 suppliers
$170.00/1g

120-92-3
427 suppliers
$11.00/25g

19980-43-9
109 suppliers
$20.00/1 g
27607-77-8
515 suppliers
$10.00/5g

120-92-3
427 suppliers
$11.00/25g

19980-43-9
109 suppliers
$20.00/1 g