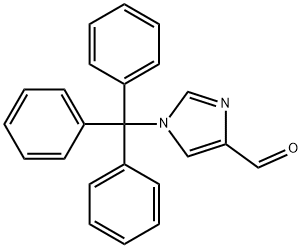
1-Tritylimidazole-4-carboxaldehyde synthesis
- Product Name:1-Tritylimidazole-4-carboxaldehyde
- CAS Number:33016-47-6
- Molecular formula:C23H18N2O
- Molecular Weight:338.4
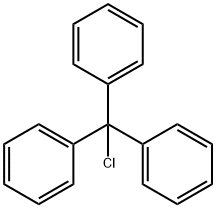
76-83-5
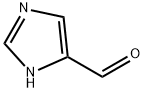
3034-50-2

33016-47-6
Example 33: Synthesis of 1-trityl-1H-imidazole-4-carbaldehyde 4-Imidazolecarboxaldehyde (30.0 g, 0.30 mol) was dissolved in DMF (200 mL) and triethylamine (70 mL, 0.375 mol) was added slowly under cooling in an ice bath. Triphenylchloromethane (105 g, 0.375 mol) was then added in batches and the reaction mixture was stirred at room temperature overnight. After completion of the reaction, the solvent was removed by vacuum evaporation and the residue was washed with anhydrous ether (4 x 50 mL) and dried to give a yellow solid 1-tritylimidazole-4-carboxaldehyde (100 g, 100%).
Yield:33016-47-6 100%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide at 0 - 20;
Steps:
33
Example 33: 1 -trityl-1 H-imidazole-4-carbaldehydeTo a solution of 1W-imidazole-4-carbaldehyde {30.0 g, 0.30 mol) in DMF (200 mL) was added Et3N (70 mL, 0.375 mol) under ice bath, and then Trt-CI (105 g, 0.375 mol) was added in portions and the mixture stirred at RT overnight. The mixture was evaporated in vacuo and the residue was washed with anhydrous Et2O (4 x 50 mL), and the resulting precipitate was dried to yield the title compound (100 g, 100%) as a yellow solid.
References:
PFIZER PRODUCTS INC. WO2008/4096, 2008, A1 Location in patent:Page/Page column 79
33769-07-2
102 suppliers
$110.00/500mg

33016-47-6
249 suppliers
$8.00/1g

76-83-5
713 suppliers
$6.00/25g

33016-47-6
249 suppliers
$8.00/1g
2591-86-8
296 suppliers
$14.00/5g
96797-15-8
242 suppliers
$21.21/250mgs:

33016-47-6
249 suppliers
$8.00/1g