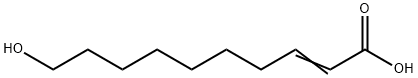
10-Hydroxy-2-decenoic acid synthesis
- Product Name:10-Hydroxy-2-decenoic acid
- CAS Number:765-01-5
- Molecular formula:C10H18O3
- Molecular Weight:186.25
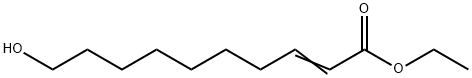
64971-15-9

765-01-5
4. Saponification reaction Dissolve 0.60 g (2.81 mmol) of the hydroxy ester obtained in the previous step in 10 volumes of tetrahydrofuran. 3.4 mL (6.75 mmol) of 2 M sodium hydroxide solution was added slowly. The reaction mixture was heated to 65 °C and kept at this temperature for 3 hours. Upon completion of the reaction, the pH of the reaction mixture was adjusted to 2 by dropwise addition of 3 M hydrochloric acid solution. the mixture was concentrated and dried, followed by extraction of the aqueous phase with ethyl acetate. The organic phase was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 0.6 g of crude product. Purification by recrystallization from cold acetonitrile afforded the target product 10-hydroxy-2-decenoic acid as a white solid in 71% yield (0.37 g). Characterization data: TLC: Rf = 0.1 (unfolding agent: heptane/ethyl acetate, 6:4, v/v) 1H NMR (300 MHz, CDCl3): δ 1.33-1.37 (m, 6H), 1.45-1.49 (m, 2H), 1.55-1.58 (m, 2H), 2.20-2.25 (q, 2H), 3.62-3.66 (t, 2H), 5.79-5.84 (d, J = 15.6 Hz, 1H), 7.03 -7.10 (dt, J = 15.6 Hz, 1H). Mass spectrum: [M-Na]+ m/z 209 (calculated value: 186) Melting point: 62.5 ± 1°C.

64971-15-9
0 suppliers
inquiry

765-01-5
145 suppliers
inquiry
Yield:765-01-5 71%
Reaction Conditions:
Stage #1:C12H22O3 with sodium carbonate in tetrahydrofuran;water at 65; for 3 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran; pH=2
Steps:
1.4
4. Saponification Reaction0.60 g (2.81 mmol) of hydroxyester obtained in the preceding step are solubilized in 10 volumes of tetrahydrofurane. 3.4 ml (6.75 mmol) of a 2M soda solution are slowly added. The medium is heated to 65° C. for 3 hours. Once the reaction is completed, the medium is hydrolyzed by adding a 3M hydrochloric acid solution, until pH=2 is obtained. The mixture is dry-concentrated and the aqueous phase is then extracted with ethyl acetate. The organic phases are washed with a NaCl saturated solution, dried on MgSO4, filtered and concentrated in vacuo in order to obtain 0.6 g of crude product.The expected unsaturated hydroxyacid is obtained, as a white solid, by recrystallization from cold acetonitrile, m=0.37 g (71%).The obtained hydroxy acid is a compound of formula (I-2) with n=6: Characterization:TLC: Rf=0.1 (heptane/ethyl acetate 6/4)1H NMR (300 MHz, CDCl3): δ 1.33-1.37 (m, 6H); 1.45-1.49 (m, 2H); 1.55-1.58 (m, 2H); 2.20-2.25 (q, 2H); 3.62-3.66 (t, 2H); 5.79-5.84 (d, J=15.6 Hz, 1H); 7.03-7.10 (dt, J=15.6 Hz, 1H).Mass spectroscopy: [M-Na]+ 209 (calculated 186)Melting point: 62.5° C.+/-1° C.
References:
Pierre Fabre Dermo-Cosmetique US2008/281114, 2008, A1 Location in patent:Page/Page column 11
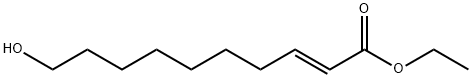
57221-93-9
4 suppliers
inquiry

765-01-5
145 suppliers
inquiry

22054-14-4
4 suppliers
inquiry

765-01-5
145 suppliers
inquiry
502-49-8
288 suppliers
$8.00/5g

765-01-5
145 suppliers
inquiry
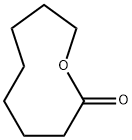
5698-29-3
3 suppliers
inquiry

765-01-5
145 suppliers
inquiry