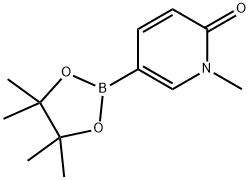
2(1H)-PYRIDINONE, 1-METHYL-5-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)- synthesis
- Product Name:2(1H)-PYRIDINONE, 1-METHYL-5-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-
- CAS Number:1002309-52-5
- Molecular formula:C12H18BNO3
- Molecular Weight:235.09
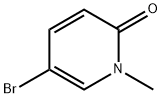
81971-39-3

73183-34-3

1002309-52-5
A dioxane (5 mL) solution of potassium acetate (270 mg, 2.67 mmol) and Pd(dppf)Cl2 (80 mg, 0.11 mmol) was added as 5-bromo-1-methylpyridin-2-one (200.0 mg, 1.06 mmol) and bis(pinacolato)diboron (410.0 mg, 1.61 mmol). The reaction mixture was microwaved at 100 °C for 2 hours. After completion of the reaction, the mixture was filtered, washed with water and extracted with ethyl acetate (20 mL x 3). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated to give the crude product 1-methyl-6-oxo-1,6-dihydropyridine-3-boronic acid pinacol ester (59.0 mg, 23.6% yield). The product was detected by LCMS and the molecular ion peak (M + H)+ was 236.
1159607-48-3
7 suppliers
inquiry

124-63-0
0 suppliers
$10.00/5g

1002309-52-5
106 suppliers
$19.00/100mg
Yield:1002309-52-5 75%
Reaction Conditions:
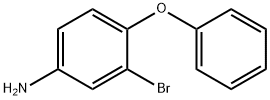
Stage #1: 3-bromo-4-phenoxyaniline;methanesulfonyl chloridewith triethylamine in dichloromethane at 20; for 1 h;
Stage #2: with sodium hydroxide in 1,4-dioxane at 70; for 1 h;
Steps:
261C 261C. N-(3-bromo-4-phenoxyphenyl)methanesulfonamide.
Example 261B (2.86 g, 10.8 mmol) and triethylamine (6.03 mL, 43.3 mmol) were stirred in dichloromethane (48.1 mL) at ambient temperature. Methanesulfonyl chloride (2.53 mL, 32.4 mmol) was added dropwise and the solution stirred at ambient temperature for 1 hour. The reaction mixture was concentrated under reduced pressure, dioxane (24 mL) and sodium hydroxide (10 % w/v, 12 mL, 0.427 mmol) were added, and the solution was heated to 70 °C for 1 hour. The solution was neutralized to a pH of 7 with saturated aqueous NH4CI (200 mL). The aqueous phase was extracted with ethyl acetate (3x125 mL). The combined organics were washed with brine, dried (MgSC^), filtered, then concentrated. The residue was purified by flash chromatography (silica gel, 0-25% ethyl acetate/hexane gradient,) to afford the title compound (2.79 g, 75%).
References:
WO2013/185284,2013,A1 Location in patent:Page/Page column 148

81971-39-3
121 suppliers
$45.00/100mg

73183-34-3
587 suppliers
$6.00/5g

1002309-52-5
106 suppliers
$19.00/100mg

1054483-78-1
105 suppliers
$25.00/100mg

74-88-4
357 suppliers
$15.00/10g

1002309-52-5
106 suppliers
$19.00/100mg

81971-39-3
121 suppliers
$45.00/100mg

73183-34-3
587 suppliers
$6.00/5g

1002309-52-5
106 suppliers
$19.00/100mg
1083169-01-0
8 suppliers
inquiry
445264-61-9
190 suppliers
$7.00/250mg

74-88-4
357 suppliers
$15.00/10g

1002309-52-5
106 suppliers
$19.00/100mg