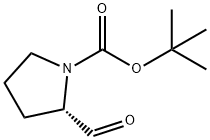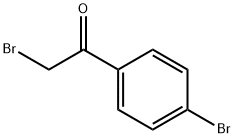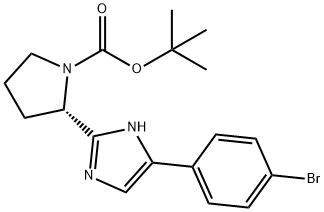
(S)-tert-butyl 2-(5-(4-bromophenyl)-1H-imidazol-2-yl)pyrrolidine-1-carboxylate synthesis
- Product Name:(S)-tert-butyl 2-(5-(4-bromophenyl)-1H-imidazol-2-yl)pyrrolidine-1-carboxylate
- CAS Number:1007882-04-3
- Molecular formula:C18H22BrN3O2
- Molecular Weight:392.29
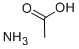
631-61-8
832 suppliers
$11.19/100G
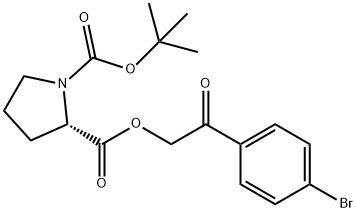
128120-93-4
1 suppliers
inquiry

1007882-04-3
60 suppliers
inquiry
Yield:1007882-04-3 141.14 g
Reaction Conditions:
in toluene at 25 - 95; for 16 h;
Steps:
1 Example-1: Preparation of (S)-tert-butyl 2-(5-(4-bromophenyl)-1H-imidazol-2-yl) pyrrolidine-1-carboxylate of formula (Va)
2-Bromo-l-(4-bromophenyl)ethanone (100 gms) was added to toluene (1000 ml) at 25-30°C and stirred for 10 min at same temperature. To the above reaction mixture, (Sj-1 - (ier/-butoxycarbonyl)pyrrolidine-2-carboxylic acid (100.67 gms) and diisopropylethylamine (106.53 gms) were added at 25-30°C. Heated the reaction mixture to 50-55°C and stirred for 4 hrs at same temperature. Cooled the reaction mixture to 25-30°C and added aqueous sodium bicarbonate solution and then stirred for 15 min. Separated the both organic and aqueous layer and added aqueous sodium chloride solution to the organic layer at 25-30°. Stirred the above reaction mixture 15 min at 25-30°C and separated the both organic and aqueous layers. (0236) Ammonium acetate (277.3 gms) was added to the above obtained organic layer at 25- 30°C Heated the reaction mixture to 90-95°C and stirred for 16 hrs at same temperature. Ethyl acetate (300 ml) and water (700 ml) were added to the reaction mixture and stirred for 15 min. Separated the both organic and aqueous layers. Washed the organic layer with aqueous sodium bicarbonate solution. Distilled off the solvent completely from the organic layer under reduced pressure and then co-distilled with cyclohexane. Cyclohexane (400 ml) and water (25 ml) were added to the above obtained material and stirred for 90 min at 25- 30°C Filtered the precipitated solid, washed with cyclohexane and then dried the obtained material to get the title compound. (0237) (Yield: 141.14 gms, % of yield: 88.4%, M.R: 162-166°C, HPLC purity: 99.4 %, bromo acetophenone: 0.01%) (0238)
References:
WO2018/134842,2018,A1 Location in patent:Page/Page column 31-32
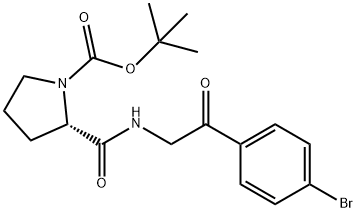
1007881-98-2
40 suppliers
inquiry

1007882-04-3
60 suppliers
inquiry

128120-93-4
1 suppliers
inquiry

1007882-04-3
60 suppliers
inquiry
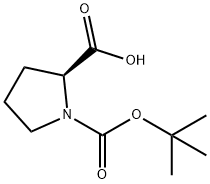
15761-39-4
608 suppliers
$5.00/10g
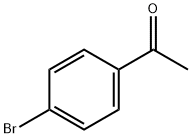
99-90-1
476 suppliers
$6.00/25g

1007882-04-3
60 suppliers
inquiry