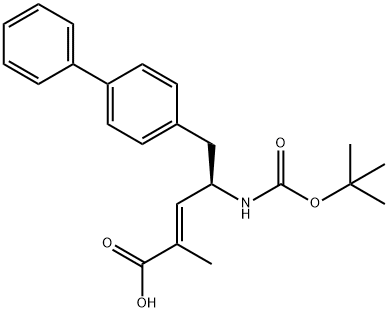
(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid synthesis
- Product Name:(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid
- CAS Number:1012341-48-8
- Molecular formula:C23H27NO4
- Molecular Weight:381.46
![(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate](/CAS/20150408/GIF/149709-59-1.gif)
149709-59-1
![(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid](/CAS/20150408/GIF/1012341-48-8.gif)
1012341-48-8
The general procedure for the synthesis of (R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methyl-2-pentenoic acid from ethyl (4R)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methyl-2-pentenoic acid was as follows: Step 1: 800 g of 95% ethanol and 400 g of pure water were mixed, compound I (1.3 mol) and lithium hydroxide (0.1833 mol) were added, and the reaction was held at reflux for 1 hour at 80 °C. After completion of the reaction, it was cooled to room temperature, activated carbon was added, heated to 42 °C and then continued to raise the temperature to 80 °C reflux. Incubate at room temperature for 2 hours. Step 2: The reaction mixture was thermally filtered and aqueous citric acid was added to the filtrate to terminate the reaction. Subsequently, the mixture was heated at reflux at 80°C for 1 hour and cooled to room temperature to crystallize. The crystals were collected by filtration and dried to give 55.4 g of dry Compound II in 79.05% molar yield and 99.20% purity.
![(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate](/CAS/20150408/GIF/149709-59-1.gif)
149709-59-1
96 suppliers
$11.00/100mg
![(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid](/CAS/20150408/GIF/1012341-48-8.gif)
1012341-48-8
223 suppliers
$9.00/1g
Yield:1012341-48-8 70%
Reaction Conditions:
with ethanol;lithium hydroxide at 35; for 1 h;
Steps:
1.6; 2.6
6) To 94.6g (4R) -5- [1,1'-biphenyl] -4-yl-4-[[tert-butoxycarbonyl] amino] -2-methyl-2-pentenoic acid ethyl esterAdd 473mL ethanolAnd 13.7g of lithium hydroxide, stirred at 35 for 1h,After the reaction, the crystals were concentrated to obtain 61.4g(R, E) -5-([1,1'-biphenyl] -4-yl) -4-((tert-butoxycarbonyl) amino) -2-methyl-2-pentenoic acid;(70% yield);
References:
NANJING HONGSHAN BIOTECHNOLOGY CO LTD;Nanjing Hongshan Biological Technology Co., Ltd.;WU FAHAO;Wu Fahao;LI GANG;Li Gang;GAO YANGZHE;Gao Yangzhe CN108299226, 2018, A Location in patent:Paragraph 0016; 0027; 0033; 0037; 0043
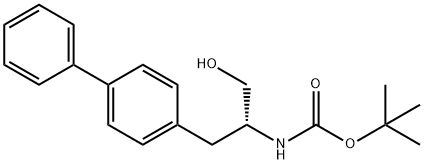
1426129-50-1
283 suppliers
$5.00/250mg
![(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid](/CAS/20150408/GIF/1012341-48-8.gif)
1012341-48-8
223 suppliers
$9.00/1g
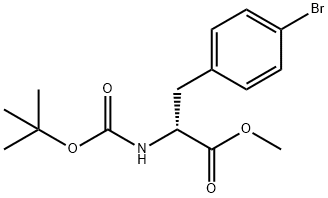
1213855-60-7
24 suppliers
$119.17/0.5g
![(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid](/CAS/20150408/GIF/1012341-48-8.gif)
1012341-48-8
223 suppliers
$9.00/1g
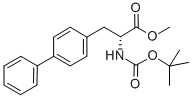
149818-98-4
25 suppliers
inquiry
![(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid](/CAS/20150408/GIF/1012341-48-8.gif)
1012341-48-8
223 suppliers
$9.00/1g
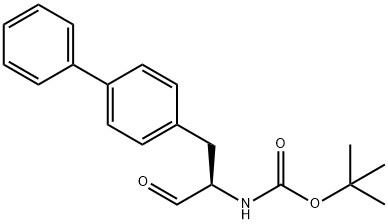
149709-58-0
46 suppliers
inquiry
![(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid](/CAS/20150408/GIF/1012341-48-8.gif)
1012341-48-8
223 suppliers
$9.00/1g