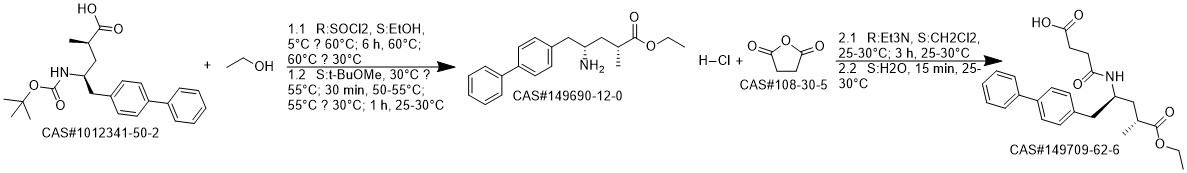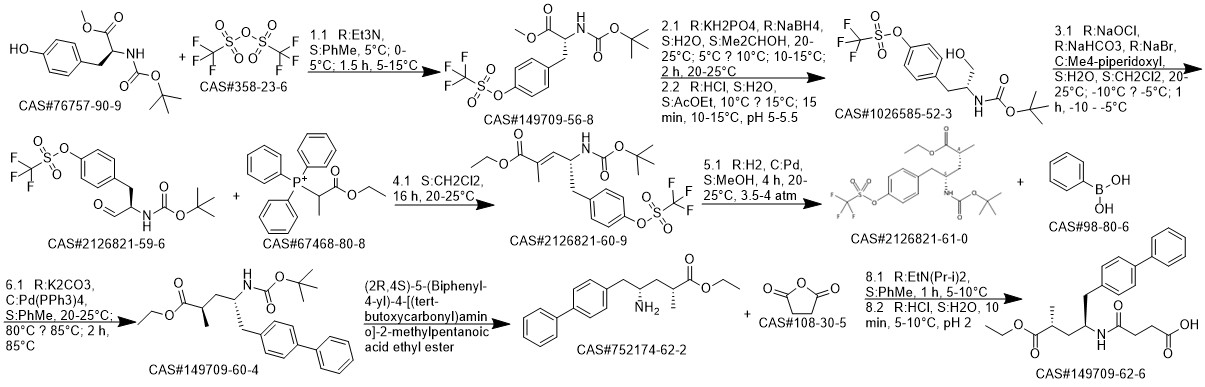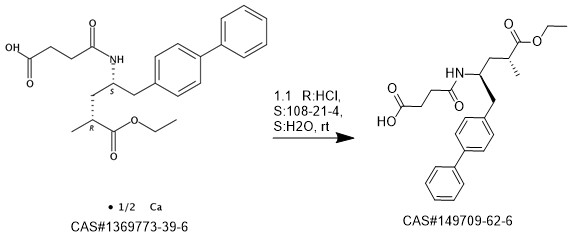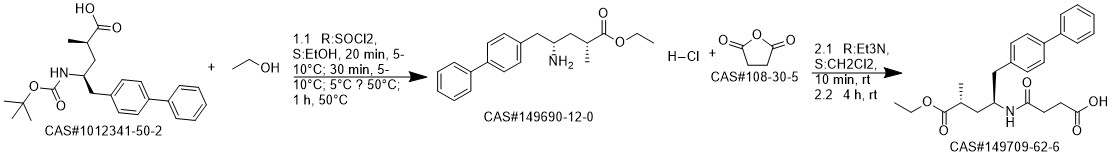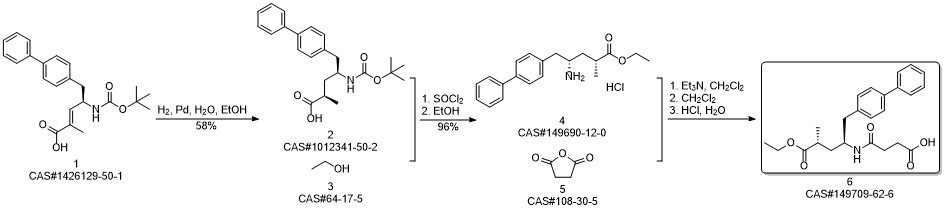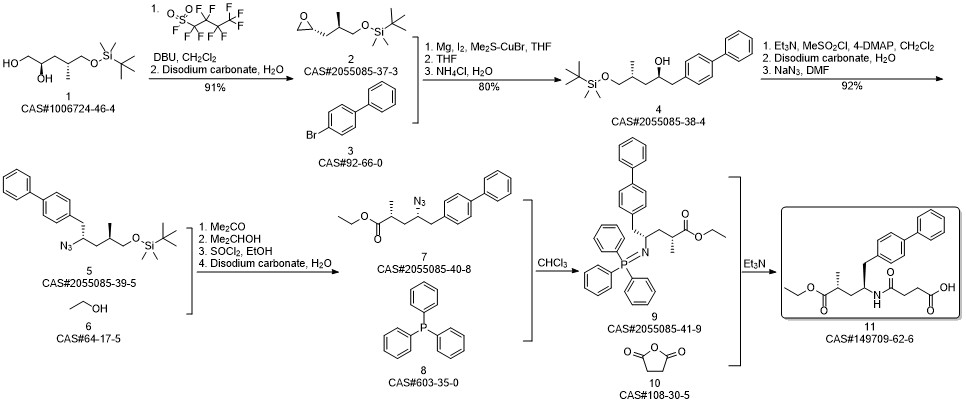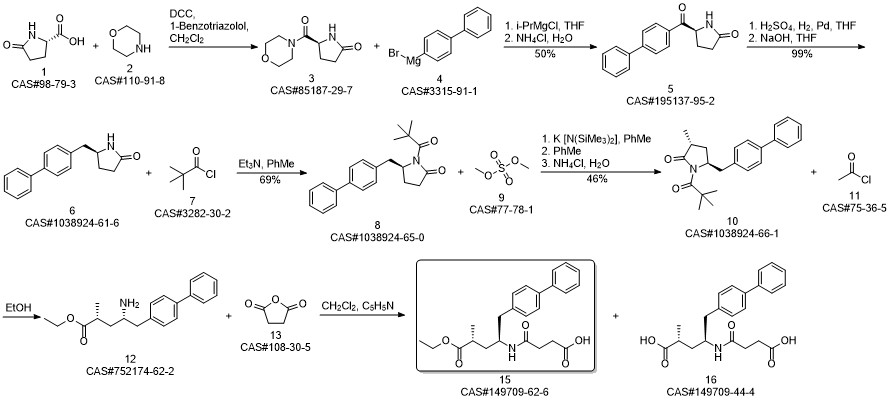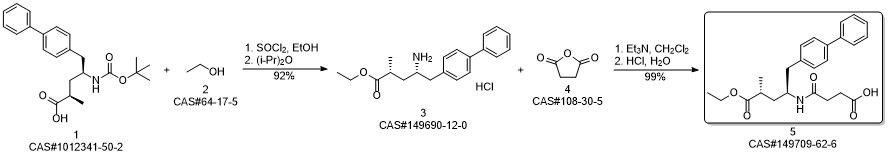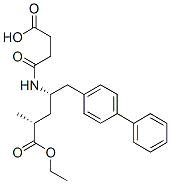
AHU-377 synthesis
- Product Name:AHU-377
- CAS Number:149709-62-6
- Molecular formula:C24H29NO5
- Molecular Weight:411.49
Reference: Zhang, Bo; Ding, Xiaohua; Dai, Dongcheng; Lei, Sijun; Liu, Xueming; Duan, Panpan; Chen, Yongkai; Wang, Chaodong. Preparation of crystal form and amorphous form of AHU-377 ammonium salt. WO 2018196860. (Wuhan Ll Science and Technology Development Co., Ltd., Peop. Rep. China; Wuhan Qr Pharmaceuticals Co., Ltd)
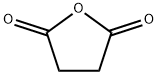
108-30-5
682 suppliers
$5.00/25g
149690-12-0
232 suppliers
$48.00/1g

149709-62-6
257 suppliers
$39.00/1mg
Yield:149709-62-6 98.5 %Chromat.
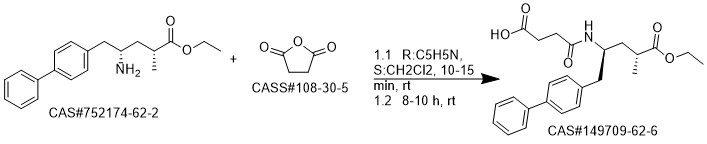
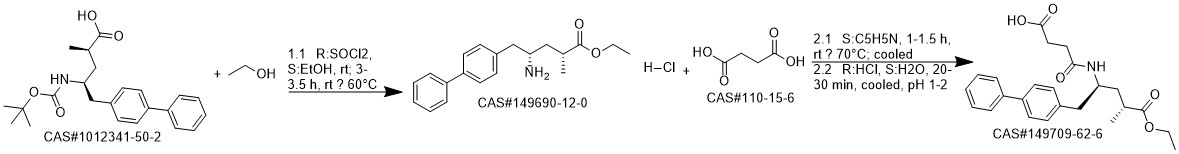
Reaction Conditions:
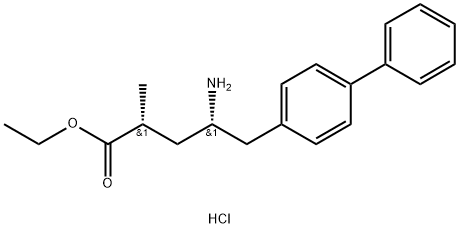
Stage #1:(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-amino-2-methylpentanoic acid ethyl ester hydrochloride with triethylamine in dichloromethane at 20; for 0.166667 h;
Stage #2:succinic acid anhydride in dichloromethane at 0; for 4 h;
Steps:
1 EXAMPLE 1 (synthesis of AHU-377 acid)
100 ml of cliehioromethane and 12 ml of triethylamine was added to 10 g of the hydrochloride of amine (3). The mixture was agitated at the room temperature for approx. 10 minutes. This was followed by addition of 4.3 g of succinic aithydride and agitation of the mixture at the room temperature for 4 hours. 100 ml of IM HCI was added to the mixture, the layers were separated, the organic layer was washed with water and dried over sodium sulphate, Afterfiltration of the desiccant the solvent was evaporated in vacuo (75°C, 10 mbar) and the product was obtained in the form of transparent honey. According to HPLC analyses the product usually contained 97.5 to 98.5% of the desired substance.
References:
ZENTIVA, K.S.;HALAMA, Ales;ZVATORA, Pavel;DAMMER, Ondrej;STACH, Jan;ZAPADLO, Michal;KREJCIK, Lukas;VOSLAR, Michal WO2016/74651, 2016, A1 Location in patent:Page/Page column 20

108-30-5
682 suppliers
$5.00/25g
![(2R,4S)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-aMino-2-Methylpentanoate](/CAS/20150408/GIF/752174-62-2.gif)
752174-62-2
34 suppliers
inquiry

149709-62-6
257 suppliers
$39.00/1mg
![Ethyl (2R,4S)-4-([1,1'-biphenyl]-4-ylmethyl)-2-methyl-4-(2,5-dioxopyrrolidin-1-yl)butanoate](/CAS/20180703/GIF/1038924-97-8.gif)
1038924-97-8
62 suppliers
inquiry

149709-62-6
257 suppliers
$39.00/1mg

108-30-5
682 suppliers
$5.00/25g
![(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-aMino-2-Methylpentanoic acid](/CAS/20150408/GIF/1039307-95-3.gif)
1039307-95-3
23 suppliers
inquiry

149709-62-6
257 suppliers
$39.00/1mg

108-30-5
682 suppliers
$5.00/25g
![(2R,4S)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoate](/CAS/20150408/GIF/149709-60-4.gif)
149709-60-4
60 suppliers
inquiry

149709-62-6
257 suppliers
$39.00/1mg