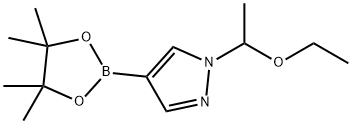
1-(1-ethoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole synthesis
- Product Name:1-(1-ethoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
- CAS Number:1029716-44-6
- Molecular formula:C13H23BN2O3
- Molecular Weight:266.14
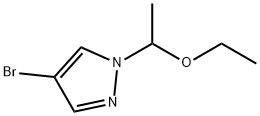
1024120-52-2
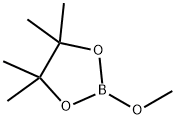
1195-66-0

1029716-44-6
Step 3: To a pre-oven-dried vial at room temperature was added a lithium isopropylmagnesium chloride solution (1.0 M, dissolved in THF, 6.32 mL, 8.22 mmol). Subsequently, 4-bromo-1-(1-ethoxyethyl)-1H-pyrazole (1.00 g, 4.56 mmol) was slowly added dropwise to this solution. The reaction mixture was stirred continuously at room temperature for 16 hours. Upon completion of the reaction, the mixture was cooled to -20 °C and 2-methoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1.73 g, 10.95 mmol) was added via syringe. Subsequently, the reaction mixture was slowly warmed up to room temperature. After continued stirring at room temperature for 2 h, the reaction was quenched by the addition of saturated aqueous ammonium chloride solution (15 mL), at which time the formation of a white precipitate was observed. The mixture was diluted with additional water (20 mL) and extracted with hexane (140 mL each time, 2 times). The organic phases were combined and washed sequentially with saturated aqueous sodium bicarbonate and brine, then dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to give 1.20 g (99% yield) of the target product 1-(1-ethoxyethyl)-4-pyrazoleboronic acid pinacol ester as a colorless oil.

1024120-52-2
76 suppliers
inquiry

1195-66-0
188 suppliers
$5.00/1g

1029716-44-6
299 suppliers
$6.00/250mg
Yield:1029716-44-6 99%
Reaction Conditions:
Stage #1: 4-bromo-1-(1-ethoxyethyl)-1H-pyrazolewith isopropyl magnesium chloride - lithium chloride complex in tetrahydrofuran at 20 - 25; for 16 h;
Stage #2: 2-methoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran at -20 - 25; for 2 h;
Steps:
18.3
Step 3[00251j To an oven-dried vial was charged a solution of isopropyl magnesium /lithium chloride solution (1.0 M in THF) (6.32 ml, 8.22 mmol) at room temperature, andto this solution was added 4-bromo-1-(1-ethoxyethyl)-1H-pyrazole (1.00 g, 4.56 mmol)dropwise and the resulting mixture was stirred at room temperature for 16 h. Theresulting solution was then cooled to -20 °C and 2-methoxy-4,4,5,5-tetramethyl-1,3,2- dioxaborolane (1.73 1 g, 10.95 mmol) was added via syringe and the resulting mixture was allowed to warm to rt. After 2h at room temperature, the reaction was quenched by addition of aq. sat. ammonium chloride (15 mL) causing a white precipitate to form.After diluting with additional water (20 mL), the mixture was extracted with hexanes (140 mL x 2) and the combined extracts were washed with aq. sat. sodium bicarbonate, brine, then dried over sodium sulfate, filtered and concentrated to afford 1.20 g (99%) of the product as a colorless oil
References:
WO2014/74661,2014,A1 Location in patent:Paragraph 00251

1024120-52-2
76 suppliers
inquiry

73183-34-3
586 suppliers
$6.00/5g

1029716-44-6
299 suppliers
$6.00/250mg
61676-62-8
324 suppliers
$11.19/5G
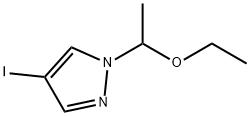
575452-22-1
67 suppliers
$9.00/100mg

1029716-44-6
299 suppliers
$6.00/250mg
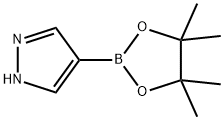
269410-08-4
397 suppliers
$5.00/1g
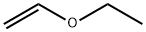
109-92-2
277 suppliers
$17.00/25mL

1029716-44-6
299 suppliers
$6.00/250mg

61676-62-8
324 suppliers
$11.19/5G

1024120-52-2
76 suppliers
inquiry

1029716-44-6
299 suppliers
$6.00/250mg