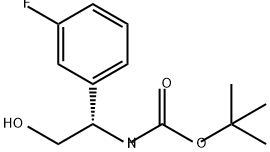
Tert-butyl (S)-(1-(3-fluorophenyl)-2-hydroxyethyl)carbamate synthesis
- Product Name:Tert-butyl (S)-(1-(3-fluorophenyl)-2-hydroxyethyl)carbamate
- CAS Number:1035490-58-4
- Molecular formula:C13H18FNO3
- Molecular Weight:255.29

24424-99-5
861 suppliers
$13.50/25G
1240480-36-7
30 suppliers
$58.00/100mg

1035490-58-4
13 suppliers
inquiry
Yield:-
Reaction Conditions:
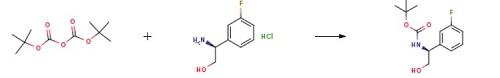
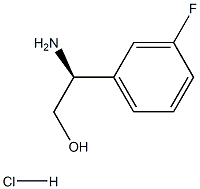
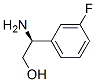
Stage #1: di-tert-butyl dicarbonate;(S)-2-amino-2-(3-fluorophenyl)ethanol hydrochloride in tert-butyl alcohol;
Stage #2: with water;sodium hydroxide in tert-butyl alcohol at 75; for 4 h;
Steps:
1
Boc-protected amino alcohol (A)A mixture of the amino alcohol hydrochloride (192.22 mmol) and di-f-butyl dicarbonate (262.63 mmol, 1.37 eq) was suspended in f-BuOH (250 mL, 6.25 volumes) and then treated with aqueous 2 N NaOH (120 mL, 240 mmol). The contents were warmed to 75 °C (immediate effervescence was observed) for 4 h. The internal temp was then reduced to 50 °C, and the contents were added to water (2 L) with vigorous stirring. After 15 min, a pure, white solid (A) precipitated, and the contents were cooled to 5 °C, prior to filtration. The collected solid was washed with water (0.5 L) and dried under vacuum at 35 °C for 18 h (90-99% yield).Example 1 was prepared following the general synthesis of A-E starting with (S)-2- amino-2-(3-fluoro-phenyl)-ethanol. LC-MS [366 (M+1 )]
References:
WO2012/69146,2012,A1 Location in patent:Page/Page column 23; 28-29

24424-99-5
861 suppliers
$13.50/25G
325152-98-5
49 suppliers
$105.00/100mg

1035490-58-4
13 suppliers
inquiry