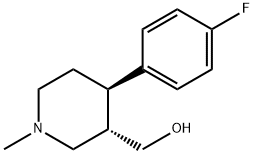
(3S,4R)-4-(4-Fluorophenyl)-3-hydroxymethyl-1-methylpiperidine synthesis
- Product Name:(3S,4R)-4-(4-Fluorophenyl)-3-hydroxymethyl-1-methylpiperidine
- CAS Number:105812-81-5
- Molecular formula:C13H18FNO
- Molecular Weight:223.29

335158-58-2

105812-81-5
The general procedure for the synthesis of (3S,4R)-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine from the compound (CAS:335158-58-2) is as follows: in a 500 mL round-bottomed flask equipped with a stirrer, a condenser and a thermometer, 31.4 g of trans-N-methyl-4'-fluorophenyl-3-methoxypiperidine and 100 mL of tetrahydrofuran were added . Subsequently, 3.31 g of lithium aluminum hydride was slowly added to the reaction system while the reaction temperature was controlled to be maintained at 10 °C. After the addition was completed, the reaction mixture was continued to be stirred until the reaction was completed. After the reaction was completed, 50 mL of distilled water and 30 mL of 10% aqueous sodium hydroxide solution were slowly added and stirring was continued for 30 minutes. Next, 200 mL of ethyl acetate was added to the reaction mixture and stirred for another 30 minutes. The organic and aqueous phases were separated and the solvent and low-boiling organic material were removed by distillation. The residue was recrystallized by a mixture of toluene and n-heptane to afford the target product trans-N-methyl-4'-fluorophenyl-3-hydroxymethylpiperidine (yield: 78.4%, purity: 99.3%). The Rf value of the product was 0.3 (unfolding agent: dichloromethane/methanol, 4:1).1H NMR (CDCl3, 200 MHz) data were as follows: δ 7.18 (t, 2H, J = 8.7 Hz); 6.98 (t, 2H, J = 8.7 Hz); 3.39 (dd, 1H, J = 10.6 Hz, J = 2.8 Hz); 3.25-3.14 ( m, 2H); 2.93 (d, 1H, J = 10.8 Hz); 2.70 (s, 1H); 2.31 (s, 3H); 2.27-2.21 (m, 1H); 2.09-1.75 (m, 5H).
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

105812-81-5
302 suppliers
$9.00/1g
Yield: 80%
Reaction Conditions:
Stage #1:(3S,4R)-3-ethoxycarbonyl-4-(4-fluorophenyl)-N-methylpiperidine-2,6-dione with lithium aluminium tetrahydride in tetrahydrofuran;toluene at 15 - 65;
Stage #2: with water;sodium hydroxide in tetrahydrofuran;toluene at 0 - 5; for 0.333333 h;
Steps:
4.3. (3S,4R)-trans-4-(4'-Fluorophenyl)-3-hydroxymethyl-N-methylpiperidine 7
Lithium aluminum hydride (1.8 g, 47.49 mmol) was added to a mixture of tetrahydrofuron (15.0 mL) and toluene (5.0 mL) at 0-5 °C and stirred for 15 min at the same temperature. A solution of (3S,4R)-3-ethoxycarbonyl-4-(4'-fluorophenyl)-N-methyl piperidine-2,6-dione 6 (5.0 g, 17.05 mmol) in toluene (15.0 mL) was added slowly for 45-60 min at below 15 °C and the mixture was heated to 60-65 °C for 2 h. The mixture was cooled to 0-5 °C and basified with 10% sodium hydroxide solution. Water (10.0 mL) was charged to the reaction mixture and then stirred for 20 min. The separated solid was filtered and washed with toluene (2 × 15 mL). The solvent was removed under reduced pressure and the crude compound was recrystalized from n-heptane to afford 7 (3.0 g, 80%) as an white solid. inlMMLBox (c 1, ethanol); IR (cm-1): 3153, 2943, 1602, 1509, 1222, 1379, 1069; 1H NMR: (400 MHz, CDCl3) δ, ppm: 7.14-7.17 (m, 2H), 6.96-7.0 (m, 2H), 3.38-3.42 (m, 1H), 3.13-3.25 (m, 2H), 2.92-2.95 (m, 1H), 2.29-2.34 (m, 4H), 1.97-2.03 (m, 5H); MS: m/z: 224.2 [M+H+].
References:
Somaiah, Sripathi;Sashikanth, Suthrapu;Raju, Veeramalla;Reddy, Karnati Venugopal [Tetrahedron Asymmetry,2011,vol. 22,# 1,p. 1 - 3] Location in patent:experimental part
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

105812-81-5
302 suppliers
$9.00/1g
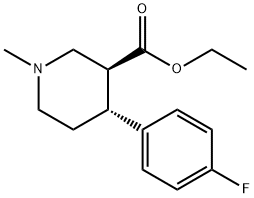
858938-15-5
0 suppliers
inquiry

105812-81-5
302 suppliers
$9.00/1g