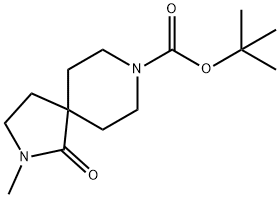
2,8-Diazaspiro[4.5]decane-8-carboxylic acid, 2-methyl-1-oxo-, 1,1-dimethylethyl ester synthesis
- Product Name:2,8-Diazaspiro[4.5]decane-8-carboxylic acid, 2-methyl-1-oxo-, 1,1-dimethylethyl ester
- CAS Number:1061683-09-7
- Molecular formula:C14H24N2O3
- Molecular Weight:268.35
![2,8-Diazaspiro[4.5]decan-1-one, 2-methyl-, hydrochloride (1:1)](/CAS/20211123/GIF/848580-34-7.gif)
848580-34-7
23 suppliers
$350.00/100mg

24424-99-5
869 suppliers
$13.50/25G
![2,8-Diazaspiro[4.5]decane-8-carboxylic acid, 2-methyl-1-oxo-, 1,1-dimethylethyl ester](/CAS2/GIF/1061683-09-7.gif)
1061683-09-7
1 suppliers
inquiry
Yield:1061683-09-7 99%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 18 h;
Steps:
10.T Method T: tert-butyl 2-methyl-1-oxo-2,8-diazaspiro[4.5]decane-8-carboxylate
Method T: tert-butyl 2-methyl-1-oxo-2,8-diazaspiro[4.5]decane-8-carboxylate A solution of 4-spiro-[3-(N-methyl-2-pyrrolidinone)]-piperidine hydrochloride (1.0 g, 5 mmol), di-tert-butyl dicarbonate (1.4 g, 6 mmol) and triethylamine (1.7 ml, 12 mmol) in dichloromethane (20 ml) was stirred at room temperature for 18 hours. The reaction mixture was diluted with dichloromethane, washed with a saturated aqueous solution of sodium bicarbonate and brine. The organic layer was dried over magnesium sulfate and concentrated under reduced pressure. The residue was purified on silica gel by flash column chromatography to afford the desired compound (1.3 g, 99% yield). 1H NMR (DMSO D6, 400 MHz) δ 1.26-1.35 (2H, m), 1.40 (9H, s), 1.52 (2H, dt), 1.91 (2H, t), 2.72 (3H, s), 2.83-2.98 (2H, m), 3.27 (2H, t), 3.77-3.87 (2H, m).
References:
US2010/137305,2010,A1 Location in patent:Page/Page column 35
![1-BOC-4-SPIRO-[3-(2-PYRROLIDINONE)] PIPERIDINE](/CAS/GIF/268550-48-7.gif)
268550-48-7
144 suppliers
$29.00/100mg

74-88-4
356 suppliers
$15.00/10g
![2,8-Diazaspiro[4.5]decane-8-carboxylic acid, 2-methyl-1-oxo-, 1,1-dimethylethyl ester](/CAS2/GIF/1061683-09-7.gif)
1061683-09-7
1 suppliers
inquiry