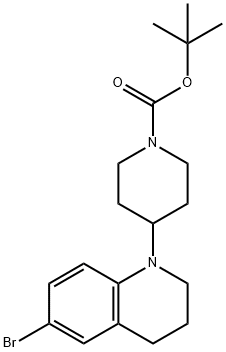
tert-Butyl 4-(6-bromo-3,4-dihydroquinolin-1(2H)-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(6-bromo-3,4-dihydroquinolin-1(2H)-yl)piperidine-1-carboxylate
- CAS Number:1063408-68-3
- Molecular formula:C19H27BrN2O2
- Molecular Weight:395.33
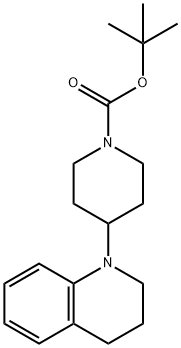
356072-30-5
6 suppliers
inquiry

1063408-68-3
8 suppliers
$655.20/5g
Yield:1063408-68-3 80.4%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 0; for 1 h;
Steps:
47
tert-butyl 4-(6-bromo-3,4-dihydroquinolin-1(2H)-yl)piperidine-1-carboxylate A solution of tert-butyl 4-(3,4-dihydroquinolin-1(2H)-yl)piperidine-1-carboxylate (0.85 g, 2.69 mmol) in 15 mL of DMF was cooled to 0° C. then treated dropwise with NBS (478 mg, 2.69 mmol) in 12 mL DMF. The reaction was stirred at 0° C. for 1 hour then treated with 100 mL H2O. The suspension was extracted with 2*75 mL of ethyl acetate. The combined organic layer was rinsed with brine (2*50 mL), dried over Na2SO4, filtered and concentrated to give a light brown oil. This residue was subjected to silica gel chromatography using the Biotage purification system (25+M column, 0-20% ethyl acetate/hexanes over 10 column volumes) to give a viscous oil which solidified to give a white solid (852 mg, 80.4%). 1H-NMR (CDCl3) δ 7.10 (dd, J=2.4 Hz, 8.7 Hz, 1H), 7.05-7.04 (m, 1H), 6.51 (d, J=9.0 Hz, 1H), 4.33-4.19 (m, 2H), 3.71-3.64 (m, 1H), 3.14 (t, J=6.0 Hz, 2H), 2.82-2.74 (m, 2H), 2.69 (t, J=6.3 Hz, 2H), 1.91-1.77 (m, 2H), 1.61-1.57 (m, 4H), 1.47 (s, 9H). MS (ESI): 395.1 and 377.1 (M+1).
References:
US2008/234237,2008,A1 Location in patent:Page/Page column 81
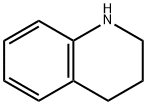
635-46-1
364 suppliers
$5.00/10g

1063408-68-3
8 suppliers
$655.20/5g

79099-07-3
0 suppliers
$5.00/5g

1063408-68-3
8 suppliers
$655.20/5g