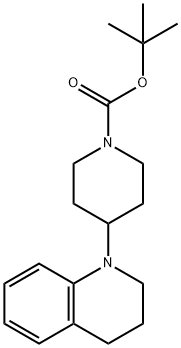
1-Piperidinecarboxylic acid, 4-(3,4-dihydro-1(2H)-quinolinyl)-, 1,1-dimethylethyl ester synthesis
- Product Name:1-Piperidinecarboxylic acid, 4-(3,4-dihydro-1(2H)-quinolinyl)-, 1,1-dimethylethyl ester
- CAS Number:356072-30-5
- Molecular formula:C19H28N2O2
- Molecular Weight:316.44
Yield:356072-30-5 37.4%
Reaction Conditions:
Stage #1: 1,2,3,4-tetrahydroisoquinoline;N-tert-butyloxycarbonylpiperidin-4-onewith sodium tris(acetoxy)borohydride;acetic acid in 1,2-dichloro-ethane at 20;
Stage #2: with sodium hydroxide;water in 1,2-dichloro-ethane at 0; for 0.333333 h;
Steps:
47
Example 47 tert-butyl 4-(3,4-dihydroquinolin-1(2H)-yl)piperidine-1-carboxylate A solution of 1,2,3,4-tetrahydroquinoline (1.06 g, 7.51 mmol) and tert-butyl 4-oxopiperidine-1-carboxylate (2.24 g, 11.26 mmol) in 20 mL 1,2-dichloroethane was treated with sodium triacetoxyborohydride (4.77 g, 22.53 mmol) then acetic acid (1.28 mL, 22.53 mmol). The suspension was stirred at room temperature overnight. At this time, a TLC analysis indicated that 1,2,3,4-tetrahydroquinoline is present along with a more polar spot. To the reaction mixture was added tert-butyl 4-oxopiperidine-1-carboxylate (1.12 g, 5.63 mmol) and sodium triacetoxyborohydride (2.39 g, 11.28 mmol). The suspension was stirred at room temperature for 4 days. After this time, the mixture was cooled to 0° C., quenched with 20 mL 1N NaOH and stirred for 20 minutes. The organic layer was separated and the aqueous layer was extracted with 50 mL CH2Cl2. The combined organic layer was dried over Na2SO4, filtered and concentrated to give a yellow residue. This residue was purified by the Biotage purification system using a silica gel 40M column. A gradient of 5% ethyl acetate:hexanes to 30% ethyl acetate:hexanes over 10 column volumes was used to give the title compound (0.89 g, 37.4%). 1H-NMR (CDCl3) δ 7.09-7.03 (m, 1H), 6.97-6.94 (m, 1H), 6.65 (d, J=8.4 Hz, 1H), 6.60-6.55 (m, 1H), 4.30-4.19 (m, 2H), 3.80-3.70 (m, 1H), 3.17 (t, J=5.7 Hz, 2H), 2.84-2.71 (m, 2H), 2.73 (t, J=6.3 Hz, 2H), 1.93-1.85 (m, 2H), 1.78-1.63 (m, 4H), 1.48 (s, 9H). MS (ESI): 317.2 (M+1).
References:
US2008/234237,2008,A1 Location in patent:Page/Page column 81
635-46-1
364 suppliers
$5.00/10g

79099-07-3
0 suppliers
$5.00/5g

144-55-8
1414 suppliers
$10.00/25g

356072-30-5
6 suppliers
inquiry