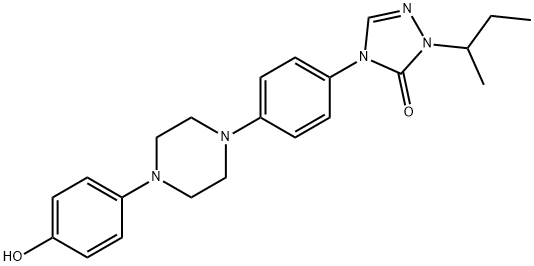
2,4-DIHYDRO-4-[(4-(4-HYDROXYPHENYL)-1-PIPERAZINYL)PHENYL]-2-(1-METHYLPROPYL)-3H-1,2,4-TRIAZOLE-3-ONE synthesis
- Product Name:2,4-DIHYDRO-4-[(4-(4-HYDROXYPHENYL)-1-PIPERAZINYL)PHENYL]-2-(1-METHYLPROPYL)-3H-1,2,4-TRIAZOLE-3-ONE
- CAS Number:106461-41-0
- Molecular formula:C22H27N5O2
- Molecular Weight:393.48
![2,4-Dihydro-4-[4-[4-(4-methoxyphenyl)-1-piperazinyl]phenyl]-2-(1-methylpropyl)-3H-1,2,4-triazol-3-one](/CAS/GIF/252964-68-4.gif)
252964-68-4
101 suppliers
$80.00/25 mg
![2,4-DIHYDRO-4-[(4-(4-HYDROXYPHENYL)-1-PIPERAZINYL)PHENYL]-2-(1-METHYLPROPYL)-3H-1,2,4-TRIAZOLE-3-ONE](/CAS/GIF/106461-41-0.gif)
106461-41-0
190 suppliers
$110.00/25 mg
Yield: 98%
Reaction Conditions:
with hydrogen bromide in water at 120;Heating / reflux;
Steps:
1.A
Triazolone 9 (0.50 g, 1.2 mmol) was added to HBr (48%, 5 mL). The reaction mixture was heated to 1200C and refluxed overnight. The reaction mixture was
References:
THE JOHNS HOPKINS UNIVERSITY WO2008/124132, 2008, A1 Location in patent:Page/Page column 49-50
![Formaldehyde, [2-(1-methylpropyl)hydrazinyl]-](/CAS/20210111/GIF/939027-86-8.gif)
939027-86-8
3 suppliers
inquiry
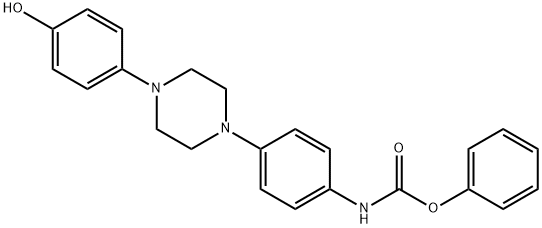
184177-81-9
200 suppliers
$70.00/1g
![2,4-DIHYDRO-4-[(4-(4-HYDROXYPHENYL)-1-PIPERAZINYL)PHENYL]-2-(1-METHYLPROPYL)-3H-1,2,4-TRIAZOLE-3-ONE](/CAS/GIF/106461-41-0.gif)
106461-41-0
190 suppliers
$110.00/25 mg
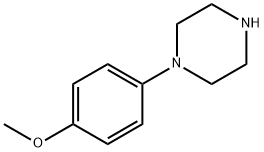
38212-30-5
205 suppliers
$15.00/5 g
![2,4-DIHYDRO-4-[(4-(4-HYDROXYPHENYL)-1-PIPERAZINYL)PHENYL]-2-(1-METHYLPROPYL)-3H-1,2,4-TRIAZOLE-3-ONE](/CAS/GIF/106461-41-0.gif)
106461-41-0
190 suppliers
$110.00/25 mg
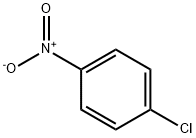
100-00-5
54 suppliers
$14.00/25g
![2,4-DIHYDRO-4-[(4-(4-HYDROXYPHENYL)-1-PIPERAZINYL)PHENYL]-2-(1-METHYLPROPYL)-3H-1,2,4-TRIAZOLE-3-ONE](/CAS/GIF/106461-41-0.gif)
106461-41-0
190 suppliers
$110.00/25 mg
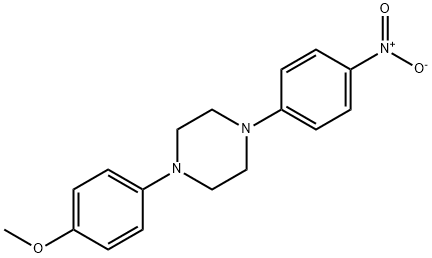
74852-61-2
135 suppliers
inquiry
![2,4-DIHYDRO-4-[(4-(4-HYDROXYPHENYL)-1-PIPERAZINYL)PHENYL]-2-(1-METHYLPROPYL)-3H-1,2,4-TRIAZOLE-3-ONE](/CAS/GIF/106461-41-0.gif)
106461-41-0
190 suppliers
$110.00/25 mg