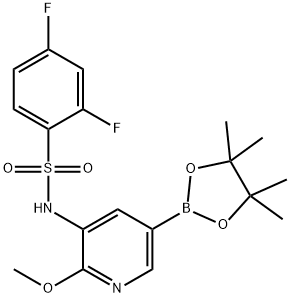
2,4-difluoro-N-(2-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-yl)benzenesulfonamide synthesis
- Product Name:2,4-difluoro-N-(2-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-yl)benzenesulfonamide
- CAS Number:1083326-73-1
- Molecular formula:C18H21BF2N2O5S
- Molecular Weight:426.24
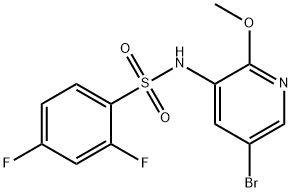
1086063-46-8
42 suppliers
inquiry

73183-34-3
587 suppliers
$6.00/5g

1083326-73-1
45 suppliers
$129.00/100mg
Yield: 100%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 90; for 7 h;Inert atmosphere;
Steps:
1.4 2,4-difluoro-N-(2-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-yl)benzenesulfonamide
To a suspension of N-(5-bromo-2-methoxypyridin-3-yl)-2,4-difluorobenzenesulfonamide (16.90 g, 44.6 mmol) in 1,4-dioxane (300 mL) was added bis(pinacolato)diboron (13.59 g, 53.5 mmol), followed by the addition of Pd(dppf)Cl2.CH2Cl2 (3.67 g, 4.5 mmol) and potassium acetate (13.12 g, 133.8 mmol). The reaction mixture was degassed with N2 for 3 times, then heated to 90° C. and stirred further for 7 hours. The mixture was then cooled to rt, quenched with H2O (100 mL) and extracted with EtOAc (500 mL×3). The combined organic phases were washed with brine (500 mL×3), dried over anhydrous Na2SO4, and concentrated in vacuo to give the title compound as a pale yellow solid (24.00 g, 100%). [0306] MS (ESI, pos. ion) m/z: 427.0 [M+H]+. [0307] 1H NMR (400 MHz, CDCl3) δ (ppm): 8.25 (d, J=1.0 Hz, 1H), 8.04 (s, 1H), 7.85 (m, 1H), 7.14 (s, 1H), 6.93 (m, 2H), 3.91 (s, 3H), 1.33 (s, 12H).
References:
SUNSHINE LAKE PHARMA CO., LTD.;CALITOR SCIENCES, LLC;Xi, Ning US2014/234254, 2014, A1 Location in patent:Paragraph 0305; 0306; 0307
![2-Methoxy-5-(4,4,5,5-tetramethyl-[1,3,2]
dioxaborolan-2-yl)-pyridin-3-ylamine](/CAS/GIF/893440-50-1.gif)
893440-50-1
185 suppliers
$25.00/1g
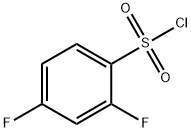
13918-92-8
202 suppliers
$5.00/1g

1083326-73-1
45 suppliers
$129.00/100mg

73183-34-3
587 suppliers
$6.00/5g

1083326-73-1
45 suppliers
$129.00/100mg
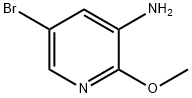
884495-39-0
172 suppliers
$5.00/1g

1083326-73-1
45 suppliers
$129.00/100mg
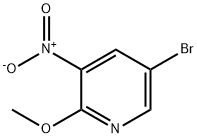
152684-30-5
221 suppliers
$6.00/1g

1083326-73-1
45 suppliers
$129.00/100mg