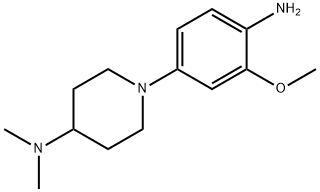
1-(4-aMino-5-Methoxy-2-Methylphenyl)-N,N-diMethylpiperidin-4-aMine synthesis
- Product Name:1-(4-aMino-5-Methoxy-2-Methylphenyl)-N,N-diMethylpiperidin-4-aMine
- CAS Number:1089279-91-3
- Molecular formula:C14H23N3O
- Molecular Weight:249.35
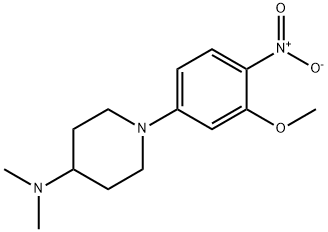
1089279-90-2

1089279-91-3
General procedure for the synthesis of 1-(4-amino-3-methoxyphenyl)-N,N-dimethylpiperidin-4-amine from BRIGATINIB intermediates: 4.09 g of Compound 29 was added to a reaction flask and dissolved with 40 mL of methanol. Subsequently, 0.4 g of 10% Pd/C catalyst (based on 10% of the mass of Compound 29) was added for the hydrogenation reduction reaction. The progress of the reaction was monitored by TLC and the reaction was complete after about 6 hours. After stopping the reaction, the reaction mixture was filtered and the catalyst was washed with a small amount of methanol. The filtrate was concentrated by distillation under reduced pressure to give 3.38 g of brown solid product 7-6 in 92.60% yield.

1089279-90-2
43 suppliers
inquiry

1089279-91-3
78 suppliers
$9.00/100mg
Yield:1089279-91-3 92.6%
Reaction Conditions:
with 10% Pd/C;hydrogen in methanol; for 6 h;
Steps:
9.4 Step (4) 1- (4-Amino-3-methoxyphenyl) -N, N-dimethylpiperidin-4-amine (7-6)
Weigh 4.09 g of compound 29 in a reaction flask,Add 40mL methanol dissolved,0.4 g of 10% Pd / C (10% by weight of II-2)Hydrogenation reduction; TLC monitoring, about 6h,Reaction is complete, stop reaction;Filter, a small amount of methanol rinse,Distillation under reduced pressure was 3.38 g of a brown solid 7-6,The yield was 92.60%.
References:
CN106905245,2017,A Location in patent:Paragraph 0249; 0259; 0260; 0261; 0262
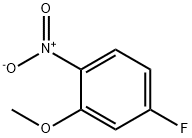
448-19-1
305 suppliers
$5.00/1g

1089279-91-3
78 suppliers
$9.00/100mg
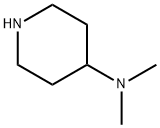
50533-97-6
190 suppliers
$11.00/1g

1089279-91-3
78 suppliers
$9.00/100mg
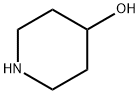
5382-16-1
0 suppliers
$9.00/5G

1089279-91-3
78 suppliers
$9.00/100mg
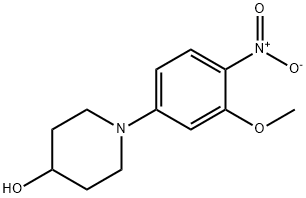
761440-22-6
21 suppliers
inquiry

1089279-91-3
78 suppliers
$9.00/100mg