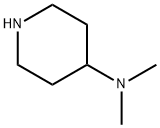
N,N-Dimethylpiperidin-4-amine synthesis
- Product Name:N,N-Dimethylpiperidin-4-amine
- CAS Number:50533-97-6
- Molecular formula:C7H16N2
- Molecular Weight:128.22
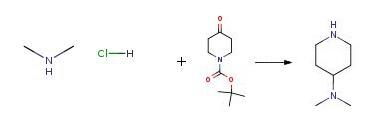
1-(tert-Butoxycarbonyl)-4-piperidone (998 mg, 4.75 mmol) in methanol (15 mL) was treated with dimethylamine hydrochloride (800 mg, 9.8 mmol) and sodium cyanoborohydride (270 mg, 4.3 mmol) at rt. After 4 days, concentrated HCl (10 mL) was added and volume of the reaction was reduced in vacuo. The resulting residue was dissolved in H2O (30 mL) and treated with a 2M NaOH solution to achieve a pH of 10. The aqueous solution was extracted with methylene chloride (3×20 mL) and the combined organics were dried over Na2SO4 and removed in vacuo. The resulting amine 106 (169 mg).
Yield:-
Reaction Conditions:
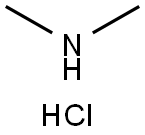
Stage #1: N,N-dimethylammonium chloride;N-tert-butyloxycarbonylpiperidin-4-onewith sodium cyanoborohydride in methanol at 20; for 96 h;
Stage #2: with hydrogenchloride in methanol;water;
Stage #3: with sodium hydroxide in water; pH=10;
Steps:
22
0300] 1-(tert-Butoxycarbonyl)-4-piperidone (998 mg, 4.75 mmol) in methanol (15 mL) was treated with dimethylamine hydrochloride (800 mg, 9.8 mmol) and sodium cyanoborohydride (270 mg, 4.3 mmol) at rt. After 4 days, concentrated HCl (10 mL) was added and volume of the reaction was reduced in vacuo. The resulting residue was dissolved in H2O (30 mL) and treated with a 2M NaOH solution to achieve a pH of 10. The aqueous solution was extracted with methylene chloride (3×20 mL) and the combined organics were dried over Na2SO4 and removed in vacuo. The resulting amine 106 (169 mg), 1H NMR (CDCl3, 300 MHz):δ3.14 (m, 2H), 2.58 (td, J=12.3, 2.4 Hz, 2H), 2.28 (s, 6H), 2.22 (m, 1H), 1.82 (m, 2H), 1.68 (s, 1H), 1.37 (tdd, J=12.2, 12.2, 4.1 Hz, 2H); ESI (+) MS: 129 (M+H+), was used without further purification.
References:
US2005/43298,2005,A1 Location in patent:Page/Page column 28-29

64168-08-7
26 suppliers
$19.00/1g

50533-97-6
191 suppliers
$11.00/1g
1122-58-3
1120 suppliers
$6.00/1g

50533-97-6
191 suppliers
$11.00/1g