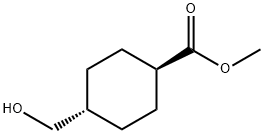
(1r,4r)-methyl 4-(hydroxymethyl)cyclohexanecarboxylate synthesis
- Product Name:(1r,4r)-methyl 4-(hydroxymethyl)cyclohexanecarboxylate
- CAS Number:110928-44-4
- Molecular formula:C9H16O3
- Molecular Weight:172.22
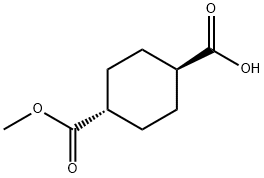
15177-67-0

110928-44-4
Tetrahydrofuran (100 mL) was cooled to -14 °C under argon protection and a tetrahydrofuran solution (100 mL) of 1.0 M borane-tetrahydrofuran complex was slowly added. Subsequently, a tetrahydrofuran solution (78 mL) of monomethyl trans-1,4-cyclohexanedicarboxylate (14.3 g) was added dropwise to this solution, and the dropwise acceleration was controlled so that the reaction was completed within 1 hour. The reaction mixture was warmed to 50 °C and stirring was continued at -10 °C for 1 hour. Upon completion of the reaction, water (160 mL) and saturated aqueous sodium bicarbonate solution (160 mL) were sequentially added to the reaction mixture under cooling in an ice bath, followed by extraction with ethyl acetate (160 mL x 4). The organic phases were combined, washed with brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product obtained was purified by fast column chromatography on silica gel (eluent: chloroform/methanol=20:1) to give methyl trans-4-(hydroxymethyl)cyclohexanecarboxylate (13.25 g, yield: 100%) as an oil. Mass spectrum (APCI) m/z: 173 [M+H]+.

15177-67-0
219 suppliers
$6.00/1g

110928-44-4
114 suppliers
$19.00/100mg
Yield: 100%
Reaction Conditions:
Stage #1:trans-4-(methoxycarbonyl)cyclohexanecarboxylic acid with borane-THF in tetrahydrofuran at -50 - -10; for 2 h;
Stage #2: with water;sodium hydrogencarbonate in tetrahydrofuran at 0;
Steps:
A.1.3 Reference Example A1
(3) Under argon gas atmosphere to a solution of momomethyl trans-cyclohexane-1,4-dicarboxylate (14.3 g) in tetrahydrofuran (78 mL) was added dropwise 1.0 M solution of borane-tetrahydrofuran complex in tetrahydrofuran (100 mL) at -50 °C over a period of 1 hour and the mixture was stirred at -10 °C for 1 hour. To the reaction mixture was added water (160 mL) and an aqueous saturated sodium hydrogencarbonate solution (160 mL) under ice-cooling and the mixture was extracted with ethyl acetate (160 mL x 4). The extract was washed with brine, dried over sodium sulfate and concentrated in vacuo. The resultant residue was purified by a flash column chromatography on silica gel (Solvent; chloroform/methanol = 20: 1) to give methyl trans-4-(hydroxymethyl)cyclohexanecarboxylate (13.25 g, yield: 100 %) as an oil. MS(APCI)m/z; 173 [M+H]+
References:
Location in patent:Page/Page column 55
![Cyclohexanecarboxylic acid, 4-[(phenylmethoxy)methyl]-, methyl ester, trans-](/CAS/20200331/GIF/1148130-27-1.gif)
1148130-27-1
0 suppliers
inquiry

110928-44-4
114 suppliers
$19.00/100mg

67-56-1
790 suppliers
$7.29/5ml-f
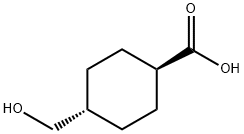
66185-74-8
68 suppliers
$92.00/100mg

110928-44-4
114 suppliers
$19.00/100mg

66185-74-8
68 suppliers
$92.00/100mg

18107-18-1
210 suppliers
$33.00/5g

110928-44-4
114 suppliers
$19.00/100mg