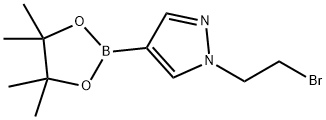
1-(2-bromoethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole synthesis
- Product Name:1-(2-bromoethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
- CAS Number:1111269-34-1
- Molecular formula:C11H18BBrN2O2
- Molecular Weight:300.99
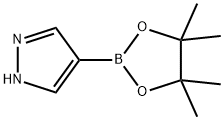
269410-08-4
398 suppliers
$5.00/1g

106-93-4
411 suppliers
$15.00/5g

1111269-34-1
12 suppliers
inquiry
Yield:1111269-34-1 45%
Reaction Conditions:
Stage #1: pyrazole-4-boronic acid pinacol esterwith potassium hydroxide in dimethyl sulfoxide at 20;
Stage #2: ethylene dibromide in dimethyl sulfoxide;
Steps:
10.A
EXAMPLE 10Part A: Potassium hydroxide (1.45 g, 10.0 equiv) was added to a stirring solution of A- (4,4,5,5-Tetramethyl-1 ,3,2-dioxaborolan-2-yl)-1H-pyrazole (500 mg, 1.00 equiv) in DMSO (5 ml_) at room temperature. After stirring for 1 hour, 1 ,2-dibromoethane (9.69 g, 20.0 equiv) was added. The reaction was stirred for 16 hours at which time TLC indicated no starting material remained so the reaction was quenched with water (10 mL) and extracted with ethyl acetate (3 x 15 mL). The combined organics were washed with brine (20 mL), dried over sodium sulfate, filtered, concentrated under reduced pressure, and purification by silica gel chromatography (12g SiO2, dichloromethane to 5% methanol in dichloromethane) afforded the desired boronate as a yellow oil 350 mg (45%).
References:
WO2009/97233,2009,A1 Location in patent:Page/Page column 83