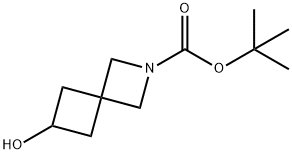
tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate synthesis
- Product Name:tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate
- CAS Number:1147557-97-8
- Molecular formula:C11H19NO3
- Molecular Weight:213.27
![tert-butyl 6-oxo-2-azaspiro[3.3]heptane-2-carboxylate](/CAS2/GIF/1181816-12-5.gif)
1181816-12-5
![tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate](/CAS2/GIF/1147557-97-8.gif)
1147557-97-8
Step 8: Synthesis of tert-butyl 6-oxo-2-azaspiro[3.3]heptane-2-carboxylate At 0 °C and under nitrogen protection, tert-butyl 6-oxo-2-azaspiro[3.3]heptane-2-carboxylate (507 mg, 2.4 mmol) was dissolved in methanol (5.0 mL) and sodium borohydride (182 mg, 4.8 mmol) was added slowly. The reaction mixture was stirred at 0 °C for 30 min. After completion of the reaction, the solvent was concentrated in vacuum by rotary evaporator to give the crude product. Saturated sodium bicarbonate solution (30 mL) was added to the crude product and the aqueous phase was extracted with dichloromethane (4 x 30 mL). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated in vacuum to afford tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate (5 mg, 100% yield) as a white solid.1H NMR (300 MHz, CDCl3): δ 4.18 (m, 1H), 3.88 (d, 4H), 2.53 (m, 2H), 2.08 (m. 2H), 1.42 (s, 9H).
![tert-butyl 6-oxo-2-azaspiro[3.3]heptane-2-carboxylate](/CAS2/GIF/1181816-12-5.gif)
1181816-12-5
190 suppliers
$9.00/100mg
![tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate](/CAS2/GIF/1147557-97-8.gif)
1147557-97-8
133 suppliers
$14.00/100mg
Yield:1147557-97-8 100%
Reaction Conditions:
with sodium tetrahydridoborate in methanol at 0; for 0.5 h;Inert atmosphere;
Steps:
1.8 Step 8 terf-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate
Step 8 terf-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate [00179] To a solution of ferf-butyl 6-oxo-2-azaspiro[3.3]heptane-2-carboxylate (507 mg, 2.4 mmol) in MeOH (5.0 mL) was added NaBH4 (182 mg, 4.8 mmol) at 0 °C under N2. It was stirred at 0 °C for 30 min. The solution was concentrated by evaporator in vacuo to give crude solid. A saturated solution of NaHCO3 (30 mL) was added. The aqueous mixture was extracted with DCM (4x30 mL). The combined organic solution was dried over anhydrous Na2SO4 and then concentrated by evaporation in vacuo to afford terf-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate (5 mg, 100%) as a white solid. [00180] 1HNMR (300 MHz, CDCI3): δ 4.18 (m, 1 H), 3.88 (d, 4 H), 2.53 (m, 2 H), 2.08 (m, 2 H), 1.42 (s, 9 H).
References:
WO2013/13308,2013,A1 Location in patent:Paragraph 00178-00180
![tert-butyl 6-(benzyloxy)-2-azaspiro[3.3]heptane-2-carboxylate](/CAS/GIF/1181816-16-9.gif)
1181816-16-9
6 suppliers
inquiry
![tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate](/CAS2/GIF/1147557-97-8.gif)
1147557-97-8
133 suppliers
$14.00/100mg
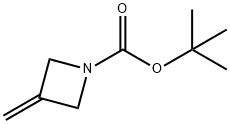
934664-41-2
111 suppliers
$20.00/1g
![tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate](/CAS2/GIF/1147557-97-8.gif)
1147557-97-8
133 suppliers
$14.00/100mg
![tert-butyl 5,5-dichloro-6-oxo-2-azaspiro[3.3]heptane-2-carboxylate](/CAS/GIF/1239320-10-5.gif)
1239320-10-5
17 suppliers
inquiry
![tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate](/CAS2/GIF/1147557-97-8.gif)
1147557-97-8
133 suppliers
$14.00/100mg