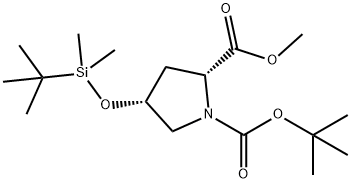
1,2-Pyrrolidinedicarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4R)- synthesis
- Product Name:1,2-Pyrrolidinedicarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4R)-
- CAS Number:114760-51-9
- Molecular formula:C17H33NO5Si
- Molecular Weight:359.53
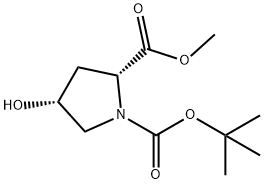
114676-69-6
187 suppliers
$6.00/1g

18162-48-6
684 suppliers
$9.00/5g
![1,2-Pyrrolidinedicarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4R)-](/CAS/20210111/GIF/114760-51-9.gif)
114760-51-9
2 suppliers
inquiry
Yield:114760-51-9 100%
Reaction Conditions:
in N,N-dimethyl-formamide at 0 - 20; for 4 h;
Steps:
1 (2R,4R)-1-tert-butyl 2-methyl 4-((tert-butyldimethylsilyl)oxy)pyrrolidine-1,2-dicarboxylate
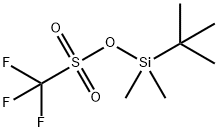
3C (49.05 g, 0.2 mol) was dissolved in N, N-dimethylformamide (300 mL) at room temperature, cooled to 0 ° C,(21.8 g, 0.22 mol) and tri-butyldimethylchlorosilane (62.7 g, 0.42 mmol) were added to the reaction mixture, and the reaction was stirred at room temperature for 4 hours.The reaction solution was poured into ice water (400 mL) and extracted with methyl tertiary butyl ether (200 mL x 3).The organic phase was washed successively with hydrochloric acid (1 mol / L, 300 mL x 1), sodium bicarbonate solution (300 mL x 1), saturated brine solution (300 mL x 2).Dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to give a pale yellow liquid 5A (72 g, yield 100%).
References:
TW2017/8221,2017,A Location in patent:Page/Page column 49
69739-34-0
368 suppliers
$10.00/1g

114676-69-6
187 suppliers
$6.00/1g
![1,2-Pyrrolidinedicarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4R)-](/CAS/20210111/GIF/114760-51-9.gif)
114760-51-9
2 suppliers
inquiry
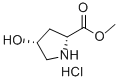
114676-59-4
113 suppliers
$6.00/250mg
![1,2-Pyrrolidinedicarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4R)-](/CAS/20210111/GIF/114760-51-9.gif)
114760-51-9
2 suppliers
inquiry
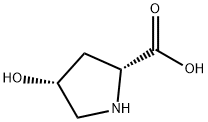
2584-71-6
338 suppliers
$6.00/1g
![1,2-Pyrrolidinedicarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4R)-](/CAS/20210111/GIF/114760-51-9.gif)
114760-51-9
2 suppliers
inquiry

24424-99-5
870 suppliers
$13.50/25G
![1,2-Pyrrolidinedicarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4R)-](/CAS/20210111/GIF/114760-51-9.gif)
114760-51-9
2 suppliers
inquiry