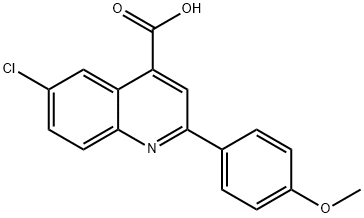
6-CHLORO-2-(4-METHOXYPHENYL)QUINOLINE-4-CARBOXYLIC ACID synthesis
- Product Name:6-CHLORO-2-(4-METHOXYPHENYL)QUINOLINE-4-CARBOXYLIC ACID
- CAS Number:116734-25-9
- Molecular formula:C17H12ClNO3
- Molecular Weight:313.74
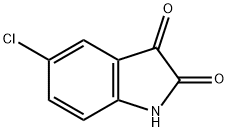
17630-76-1
290 suppliers
$6.00/5g
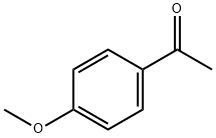
100-06-1
611 suppliers
$18.19/25g

116734-25-9
12 suppliers
inquiry
Yield:116734-25-9 66%
Reaction Conditions:
Stage #1: 5-chloroindole 2,3-dionewith potassium hydroxide for 0.5 h;Reflux;Pfitzinger reaction;
Stage #2: 1-(4-methoxyphenyl)ethanone for 20 h;Reflux;Pfitzinger reaction;
Steps:
5.1.2.1. 6-Chloro-2-(4-methoxyphenyl) quinoline-4-carboxylic acid (2a)
5-Chloroindoline-2, 3-dione (0.908 g, 5 mmol) was weighed into a round-bottom flask and 4 mL, 33% potassium hydroxide solution was added. The reaction mixture was refluxed with magnetic stirring for 0.5 h and cooled to 80 °C. Then 1-(4-methoxyphenyl) ethanone (0.9 g, 65 mmol) was added, refluxed for 20 h, and cooled to room temperature. Then 200 mL water was added to the mixture, and extracted twice with ether; the water layer was acidified by slowly adding 6 M hydrochloric acid to pH 3. A light yellow solid formed. The product was collected by suction filtration and air-dried. The crude product (2a) was obtained at 66% yield.
References:
Wu, Yiran;Chen, Zheng;Liu, Ying;Yu, Lanlan;Zhou, Lu;Yang, Suijia;Lai, Luhua [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 11,p. 3361 - 3366] Location in patent:experimental part