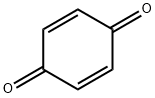
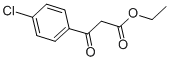
ethyl 2-(4-chlorophenyl)-5-hydroxybenzofuran-3-carboxylate synthesis
- Product Name:ethyl 2-(4-chlorophenyl)-5-hydroxybenzofuran-3-carboxylate
- CAS Number:1192977-48-2
- Molecular formula:C17H13ClO4
- Molecular Weight:316.74
Yield:1192977-48-2 43.9%
Reaction Conditions:
with zinc(II) chloride in ethanol;tert-butyl methyl ether at 75 - 95; for 2 h;Inert atmosphere;
Steps:
34.2
Step 2: ethyl 2-(4-chlorophenyl)-5-hydroxybenzofuran-3-carboxylateZinc chloride (28.3 g, 0.207 mol) was stirred in anhydrous ethanol (45 mL) then heated to 95 °C (reflux) under nitrogen atmosphere using oven dried glassware. Ethyl 4- Chlorobenzoylacetate (44 g, 0.194 mol) was added in a single portion followed by dropwise addition of a solution of benzoquinone (22.6 g, 0.21 mol) in anhydrous MTBE (500 mL) over 2 hours. This was performed with a simultaneous distillation of MTBE from the reaction mixture such that the reaction volume remained approximately constant. A bath temperature of 145-155 °C and an internal temperature of 75-95 °C maintained throughout most of the addition. Half way through the addition more anhydrous ethanol (45 ml.) was added because the reaction mixture became thick and a loss of some of the orginal volume of ethanol through the distillation was suspected. After addition was complete, heating continued for 30 minutes. The reaction mixture was cooled to room temperature and partitioned between water (100 ml.) and EtOAc (250 ml_). The insoluble solids were removed by filtration of the biphasic solution and the organic layer was then separated, washed with more water, dried and evaporated under vacuum. The residual brown solid was slurried in warmdichloromethane and the mixture cooled to room temperature and cooled further by refrigeration overnight. The tan solid was filtered from the dark brown solution and washed with a small volume of DCM and dried under vacuum to give the benzofuran (27 g, 43.9 %).
References:
WO2012/67663,2012,A1 Location in patent:Page/Page column 91-92
122-01-0
384 suppliers
$13.00/25g

1192977-48-2
6 suppliers
inquiry
74-11-3
605 suppliers
$5.00/1g

1192977-48-2
6 suppliers
inquiry