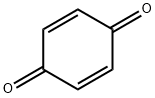
1,4-Benzoquinone synthesis
- Product Name:1,4-Benzoquinone
- CAS Number:106-51-4
- Molecular formula:C6H4O2
- Molecular Weight:108.09
Principle: Hydroquinone can be readily oxidized to quinone by using oxidizing agent like potassium dichromate and conc. H2SO4 or KBrO3.
Reaction:
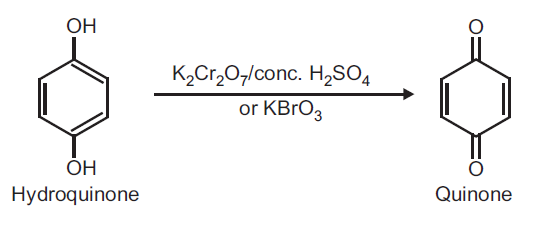
Procedure: Take 0.5 g hydroquinone and 5 ml distilled water in a beaker (25 ml). Heat on a wire gauze to obtain a clear solution. Take 1 g potassium dichromate (K2Cr2O7) in a conical flask and dissolve in 10 ml water and add 1 ml conc. H2SO4. Shake and cool the conical flask in ice water. To this ice cold solution add hydroquinone solution (prepared above) dropwise over a period of 30 minutes with constant shaking. Do not allow the temperature to rise above 20oC. After complete addition, continue shaking for further 10 minutes. Yellow crystals of quinone separate out. Filter on a Buchner funnel and dry it well. (Note: Do not wash with water as the product is water soluble). Record the practical yield and re-crystallize from ethyl alcohol.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. The yellow needles of quinone separate out. Filter, dry and record the melting point and TLC (using toluene as a solvent).
1165952-92-0
0 suppliers
inquiry

106-51-4
494 suppliers
$10.00/5g
Yield:106-51-4 83%
Reaction Conditions:
with tert.-butylhydroperoxide;Cu[PHMAPM];dihydrogen peroxide in acetonitrile at 70; for 6 h;Reagent/catalyst;Time;Solvent;
Steps:
Catalytic activity studies: General procedure for allylicoxidation
General procedure: To a solution of Co(II)/Ni(II)/Cu(II) complex (1.25 μmol), corresponding diene (0.5 mmol) and H2O2/TBHP (2.0 mmol) in 10 mL of acetonitrile was added. After the reaction was stirred at 70 °C and time as given in Table 1, (reaction monitored by LC-MS), the solvent was removed under reduced pressure. The residue was purified by flash column chromatography with hexane/dichloromethane (3:1, v/v) as eluent to afforded pure product. The 1H NMR spectra of the products are given under Supplementary Data (Figs S1-S7).
References:
Jyothi;Rao, G. Raghava;Shashank;Sridhar;Reddy, Anjali;Someshwar;Swamy, S. Jagannatha [Indian Journal of Chemistry, Section A: Inorganic, Physical, Theoretical and Analytical,2014,vol. 53,# 4-5,p. 535 - 544]
3424-93-9
209 suppliers
$14.00/1g

106-51-4
494 suppliers
$10.00/5g
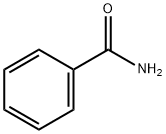
55-21-0
412 suppliers
$5.00/5G

106-51-4
494 suppliers
$10.00/5g
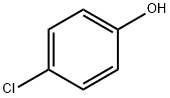
106-48-9
498 suppliers
$10.00/5G

106-51-4
494 suppliers
$10.00/5g
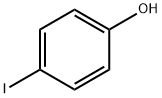
540-38-5
347 suppliers
$10.00/1g

106-51-4
494 suppliers
$10.00/5g