
1-(4-(1-CYANOCYCLOBUTYL)PHENYL)-4-METHOXY-2-OXO-1,2,5,6-TETRAHYDROPYRIDINE-3-CARBONITRILE synthesis
- Product Name:1-(4-(1-CYANOCYCLOBUTYL)PHENYL)-4-METHOXY-2-OXO-1,2,5,6-TETRAHYDROPYRIDINE-3-CARBONITRILE
- CAS Number:1236409-75-8
- Molecular formula:C18H17N3O2
- Molecular Weight:307.35
Yield:1236409-75-8 92%
Reaction Conditions:
Stage #1: 1-(4-(1-cyanocyclobutyl)phenyl)-4-hydroxy-2-oxo-1,2,5,6-tetrahydropyridine-3-carbonitrilewith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 2.25 h;
Stage #2: methanol in dichloromethane at 45; for 16 h;
Steps:
1-(4-(1-Cyanocyclobutyl)phenyl)-4-hydroxy-2-oxo-1,2,5,6-tetrahydropyridine-3-carbonitrile (50.0 g, 170 mmol) and N,N-dimethylformamide (0.66 mL, 8.5 mmol) in dichloromethane (350 mL) was cooled to 0° C. Oxalyl chloride (18.0 mL, 203 mmol) was added over 15 minutes. The mixture was warmed to room temperature over 2 hours. Methanol (300 mL) was then added as a steady stream, and the mixture was heated at 45° C. for 16 hours. The mixture was cooled to room temperature and concentrated to get rid of most of the dichloromethane. Methanol (200 mL) was added and the thick slurry was stirred for 2 hours. The solid was filtered and dried under vacuum to give 1-(4-(1-cyanocyclobutyl)phenyl)-4-methoxy-2-oxo-1,2,5,6-tetrahydropyridine-3-carbonitrile (48.3 g, 92%) as an off-white powder. 1H NMR (400 MHz, DMSO-d6) δ ppm 1.91-2.03 (m, 1H) 2.18-2.31 (m, 1H) 2.54-2.63 (m, 2H) 2.67-2.75 (m, 2H) 3.03 (t, J=6.73 Hz, 2H) 3.85 (t, J=6.73 Hz, 2H) 4.01 (s, 3H) 7.33 (d, J=8.78 Hz, 2H) 7.44 (d, J=8.78 Hz, 2H) m/z (M+1)=308.4
References:
US2010/197591,2010,A1 Location in patent:Page/Page column 32

811803-25-5
23 suppliers
inquiry

1236409-75-8
4 suppliers
inquiry
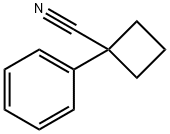
14377-68-5
117 suppliers
$14.00/1g

1236409-75-8
4 suppliers
inquiry
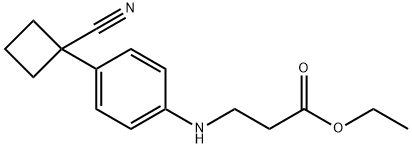
1236409-70-3
1 suppliers
inquiry

1236409-75-8
4 suppliers
inquiry
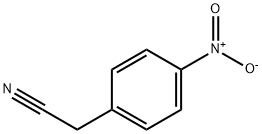
555-21-5
68 suppliers
$21.00/25g

1236409-75-8
4 suppliers
inquiry

