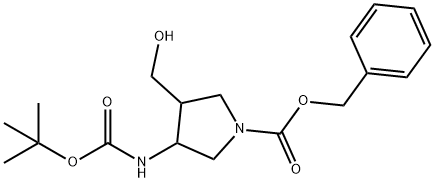
3-(N-tert-butoxycarbonyl)amino-4-hydroxymethyl-N-benzyloxycarbonylpyrrolidine synthesis
- Product Name:3-(N-tert-butoxycarbonyl)amino-4-hydroxymethyl-N-benzyloxycarbonylpyrrolidine
- CAS Number:1255099-67-2
- Molecular formula:C18H26N2O5
- Molecular Weight:350.41

24424-99-5
861 suppliers
$13.50/25G

1017789-40-0
17 suppliers
$90.00/50mg

1255099-67-2
23 suppliers
$65.00/10mg
Yield:1255099-67-2 64%
Reaction Conditions:
with sodium hydrogencarbonate in ethanol;pentan-1-ol;water at 20; for 15 h;Inert atmosphere;
Steps:
2.F
Step F. 3-te/t-Butoxycarbonylamino-4-hydroxymethyl-pyrrolidine-1 - carboxylic acid benzyl ester. To a 3L round-bottomed flask, was added the filtered reaction mixture of 3-amino-4-hydroxymethyl-pyrrolidine-1 -carboxylic acid benzyl ester in pentanol/ethanol mixture. To the mixture sodiumbicarbonate (30.8 g, 367 mmol), H2O (655 mL), and Boc anhydride (38 g, 174.1 mmol) were added. The mixture was stirred under N2(9) for 15 h at room temperature. The biphasic mixture was then separated and the organics were washed with brine (655 mL). The organics were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The material was warmed to 98 C in toluene/heptanes (280 mL each) and then filtered. The filtrate was slowly cooled to room temeperature and stirred for 20 h. The resulting solids were filtered and washed with heptanes to provide the title compound (39.5 g, 64% over two steps). 1H-NMR (CD3OD, 400 MHz): 7.31 (m, 5H), 5.1 1 (s, 2H), 4.20 (m, 1 H), 3.50-3.70 (m, 4H), 3.34-3.42 (m, 1 H), 3.18-3.29 (m, 1 H), 2.49 (m, 1 H), 1 .44 (s, 9H).
References:
WO2011/50202,2011,A1 Location in patent:Page/Page column 48-49
501-53-1
407 suppliers
$10.00/1g

1255099-67-2
23 suppliers
$65.00/10mg

114790-39-5
49 suppliers
$25.00/100mg

1255099-67-2
23 suppliers
$65.00/10mg
569682-60-6
26 suppliers
inquiry

1255099-67-2
23 suppliers
$65.00/10mg

370880-75-4
34 suppliers
$206.00/1g

1255099-67-2
23 suppliers
$65.00/10mg