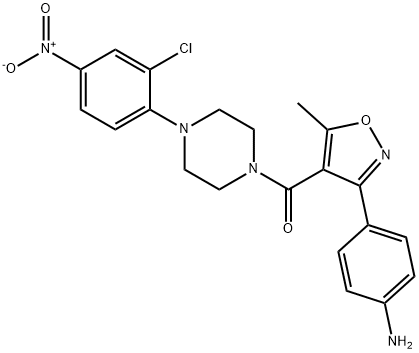
(3-(4-aMinophenyl)-5-Methylisoxazol-4-yl)(4-(2-chloro-4-nitrophenyl)piperazin-1-yl)Methanone synthesis
- Product Name:(3-(4-aMinophenyl)-5-Methylisoxazol-4-yl)(4-(2-chloro-4-nitrophenyl)piperazin-1-yl)Methanone
- CAS Number:1264870-22-5
- Molecular formula:C21H20ClN5O4
- Molecular Weight:441.87

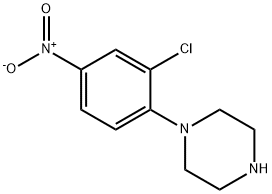
7035-83-8
7 suppliers
inquiry
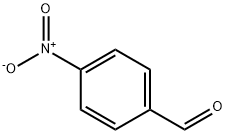
114878-60-3
101 suppliers
$12.00/250mg

1264870-22-5
5 suppliers
inquiry
Yield:1264870-22-5 49%
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 4 h;
Steps:
3
Synthesis of Compound 3 (YD-03)To a solution of crude YD-038 (157 mg, 0.72 mmol) in anhydrous dichloromethane (8 mL) were added sequentially N,N-diisopropylethylamine (DIEA, 138 mg, 1.08 mmol), YD-05 (207 mg, 0.86 mmol) and EDCI (550 mg, 2.88 mmol). The resulting mixture was stirred at room temperature for 4 hours and then diluted with dichloromethane. This mixture was washed with 2 M sodium hydroxide solution, water and saturated brine. The organic layer was dried over sodium sulfate, filtrated and concentrated. The residue obtained was purified by column chromatography (petroleum ethe?ethyl acetate = 2:1) affording a bright yellow powder of YD-03 (173 mg, 49%). 1H NMR (400 MHz, DMSO-d6), δ 8.24 (d, J = 2.7 Hz, 1 H, 1 '-H), 8.15 (dd, J = 9, 2.7 Hz, 1 H, 2'-H), 7.28 (d, J = 8.6 Hz, 2 H, 2-H, 3-H), 7.19 (d, J = 9 Hz, 1 H, 3'-H), 6.63 (d, J = 8.6 Hz, 2 H, 1-H, 4-H), 5.60 (br s, 2 H, NH2), 3.81 (br s, 2 H, CH2), 3.34 (br s, 2 H, CH2), 3.20 (br s, 2 H, CH2), 2.86 (br s, 2 H, CH2), 2.42 (s, 3 H, -CH3);13C NMR (100 MHz, DMSO-d6), δ 167.9, 161.8, 159.5, 154.0, 150.7, 141.9, 128.2, 126.4, 125.9, 123.7, 120.6, 114.5, 113.7, 110.2, 50.0, 49.8, 46.3, 41.2, 11.4; LRMS (API-ES): 442 (M++ H).
References:
WO2011/15037,2011,A1 Location in patent:Page/Page column 42; 54-55
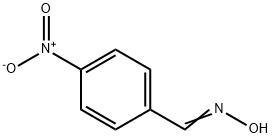
555-16-8
574 suppliers
$5.00/5g

1264870-22-5
5 suppliers
inquiry
1129-37-9
100 suppliers
$18.00/1g

1264870-22-5
5 suppliers
inquiry
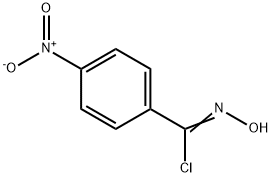
1011-84-3
42 suppliers
$38.00/1/ G

1264870-22-5
5 suppliers
inquiry
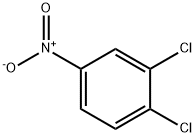
99-54-7
33 suppliers
$13.00/25g

1264870-22-5
5 suppliers
inquiry