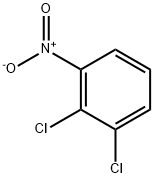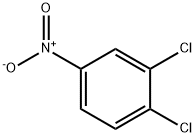
3,4-Dichloronitrobenzene synthesis
- Product Name:3,4-Dichloronitrobenzene
- CAS Number:99-54-7
- Molecular formula:C6H3Cl2NO2
- Molecular Weight:192
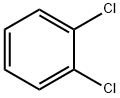
95-50-1
601 suppliers
$10.00/5g

99-54-7
33 suppliers
$13.00/25g
Yield:99-54-7 98%
Reaction Conditions:
with sulfuric acid;nitric acid in water at 70;Temperature;Reagent/catalyst;
Steps:
1-56
General procedure: When working, feed raw material 1 (ODCB mixed with catalyst), raw material 2 (concentrated sulfuric acid), and raw material 3 (concentrated nitric acid) to the full continuous flow reaction system with a constant flow pump. The feed rate is shown in the following tables. Feed rate 1 represents the feed rate of raw material 1, feed rate 2 represents the feed rate of raw material 2, and feed rate 3 represents the feed rate of raw material 3. Enter the temperature zone 1 and stir and mix until the nitration reaction in the temperature zone 2, and the reaction is complete; the reaction liquid out of the temperature zone 2 enters the temperature zone 3 and the temperature zone 4 for post-processing to obtain the product. Among them, the process temperature is controlled in the unit of temperature zone, and the reaction time is controlled by the ratio of the flow time of each module and the total continuous flow synthesis process time. The time ratio of each module is: module 1: module 2: module 3 : Module 4: Module 5 is 5:4:4:5:5, and the full continuous flow synthesis process time of each embodiment is shown in the following tables. The various process parameters of the specific embodiments are shown in the following tables, where the amount of catalyst refers to the mass percentage of the catalyst and the reaction substrate.
References:
Zhejiang Ben Li Technology Co., Ltd.;Shu Xinlin;Li Jianmin;Wu Zhengjie CN111116372, 2020, A Location in patent:Paragraph 0070-0082
95-76-1
377 suppliers
$10.00/5g

99-54-7
33 suppliers
$13.00/25g
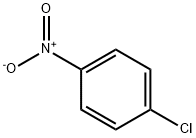
100-00-5
55 suppliers
$14.00/25g

99-54-7
33 suppliers
$13.00/25g
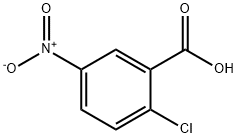
2516-96-3
453 suppliers
$6.00/25g

99-54-7
33 suppliers
$13.00/25g