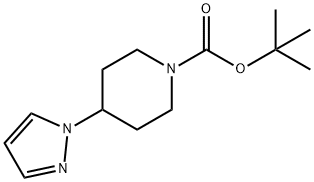
tert-butyl 4-(1H-pyrazol-1-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(1H-pyrazol-1-yl)piperidine-1-carboxylate
- CAS Number:1269429-29-9
- Molecular formula:C13H21N3O2
- Molecular Weight:251.32
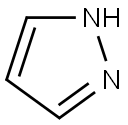
288-13-1
451 suppliers
$10.00/1g
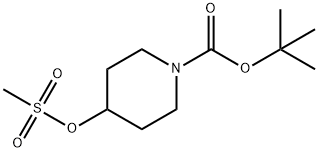
141699-59-4
230 suppliers
$6.00/1g

1269429-29-9
25 suppliers
inquiry
Yield:1269429-29-9 55.9%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 50; for 12 h;
Steps:
3A1 synthesis of tert-butyl 4-( lH-pyrazol- 1 -yl)piperidine- 1 -carboxylate
Example 3[(2S,3aR,6aR)-2-[4-(lH-pyrazol- 1 -yl)piperidin- 1 -yl]hexahydropentalen-3a(lH)-yl] [3- (trifluoromethyl)-7,8-dihydro-l,6-naphthyridin-6(5H)-yl]methanone Example 3A1tert-butyl 4-( lH-pyrazol- 1 -yl)piperidine- 1 -carboxylate To a solution of lH-pyrazole (0.51 g, 7.46 mmol) in DMF (20 mL) was added NaH (0.43 g, 10.67 mmol) and Example 4A (2 g, 7.1 1 mmol) at room temperature. The mixture was stirred for 12 hours at 50 °C. TLC (petroleum ethenEtOAc = 1 : 1) indicated the reaction was completed. The reaction mixture was quenched with aqueous NH4CI4 (20 mL) and extracted with EtOAc (3X 50mL). The organic layer was dried over Na2S04, filtered, and concentrated in vacuum, the residue was purified by silica gelchromatography to give the title compound (1.0 g, 55.9%) as a colorless solid. NMR (400 MHz, CDCI3) δ 7.45 (d, IH), 7.35 (d, IH), 6.19 (t, IH), 4.21 (m, 3H), 2.82 (m, 2H), 2.03 (m, 2H), 1.84 (m, 2H), 1.40 (s, 9H).
References:
WO2013/10453,2013,A1 Location in patent:Page/Page column 95
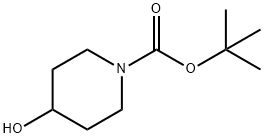
109384-19-2
434 suppliers
$5.00/1g

1269429-29-9
25 suppliers
inquiry