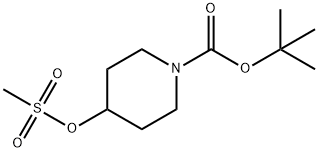
1-Boc-4-methanesulfonyloxypiperidine synthesis
- Product Name:1-Boc-4-methanesulfonyloxypiperidine
- CAS Number:141699-59-4
- Molecular formula:C11H21NO5S
- Molecular Weight:279.35

124-63-0
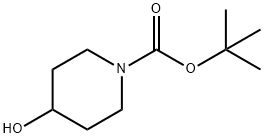
109384-19-2

141699-59-4
N-Boc-4-hydroxypiperidine (10 g, 49.68 mmol, 1 eq.) and triethylamine (7.54 g, 74.50 mmol, 1.5 eq., 10.38 ml) were dissolved in anhydrous dichloromethane (200 ml) and cooled to 0-5 °C. Methylsulfonyl chloride (6.88 g, 60.12 mmol, 4.65 ml, 1.21 eq.) was added slowly and dropwise under stirring. The reaction mixture was stirred at 0-5 °C for 3 hours. After completion of the reaction, the mixture was diluted with dichloromethane (200 ml), transferred to a separatory funnel and washed sequentially with 2M aqueous sodium carbonate solution (200 ml) and saturated sodium chloride solution (200 ml). The organic layer was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 14.51 g of tert-butyl 4-((methylsulfonyl)oxy)piperidine-1-carboxylate as a white solid (yield = 100%). The product was confirmed by 1H-NMR (400 MHz, DMSO-d6) and mass spectrometry (EI): δ 4.80 (m, 1H), 3.39 (m, 2H), 3.29 (m, 2H), 2.94 (s, 3H), 1.77 (m, 2H), 1.52 (m, 2H); MS (EI): 280 (MH+).

124-63-0
0 suppliers
$10.00/5g

109384-19-2
420 suppliers
$5.00/1g

141699-59-4
229 suppliers
$6.00/1g
Yield:141699-59-4 100%
Reaction Conditions:
with triethylamine in dichloromethane;ethyl acetate
Steps:
14.B Preparation of N-hydroxy-1-(phenyl-methyl)-4-[[4-[4-(trifluoromethoxy)-phenoxy]-1-piperidinyl]sulfonyl]-4-piperidinecarboxamide, monohydrochloride
Part B: Preparation of 4-(methylsulfonyl)hydroxy-1-piperidinecarboxylic acid, 1,1-dimethylethyl ester. To a solution of the BOC piperidine of part A (5.00 g, 24.84 mmol) in dichloromethane (50 mL) at 0° C., was added triethylamine (3.81 mL, 27.32 mmol) followed by methane sulfonyl chloride (2.02 mL, 26.08 mmol). Once the addition was complete the cooling bath was removed. After stirring for two hr the reaction mixture was concentrated in vacuo. The residue was taken up in ethyl acetate, washed with water two times, saturated sodium chloride solution, dried over Na2SO4, filtered, and concentrated in vacuo to provide the mesylate as an off-white solid (7.34 g, >100%).
References:
G. D. Searle & Company US6372758, 2002, B1

124-63-0
0 suppliers
$10.00/5g

109384-19-2
420 suppliers
$5.00/1g

141699-59-4
229 suppliers
$6.00/1g
5382-16-1
0 suppliers
$9.00/5 g

24424-99-5
869 suppliers
$13.50/25G

124-63-0
0 suppliers
$10.00/5g

141699-59-4
229 suppliers
$6.00/1g
37595-74-7
436 suppliers
$5.00/1g

141699-59-4
229 suppliers
$6.00/1g

124-63-0
0 suppliers
$10.00/5g

109384-19-2
420 suppliers
$5.00/1g
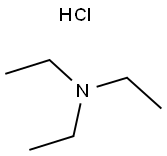
554-68-7
503 suppliers
$6.00/25g

141699-59-4
229 suppliers
$6.00/1g