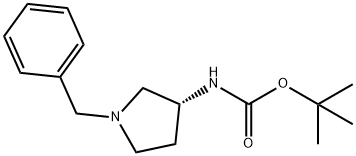
(3R)-(+)-1-BENZYL-3-(TERT-BUTOXYCARBONYLAMINO)PYRROLIDINE synthesis
- Product Name:(3R)-(+)-1-BENZYL-3-(TERT-BUTOXYCARBONYLAMINO)PYRROLIDINE
- CAS Number:131878-23-4
- Molecular formula:C16H24N2O2
- Molecular Weight:276.37

24424-99-5
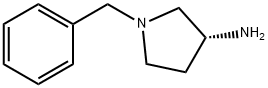
114715-39-8

131878-23-4
The general procedure for the synthesis of (R)-1-benzyl-3-(tert-butoxycarbonylamino)pyrrolidine from di-tert-butyl dicarbonate and (R)-1-benzyl-3-amino pyrrolidine was as follows: first, sodium bicarbonate (5.92 g, 70.5 mmol) was dissolved in 118 mL of deionized water, and then this solution was added to a 118 mL acetonitrile solution of (R)-(+)-benzylamino pyrrolidine 1a (5.00 g, 28.4 mmol) in 118 mL of acetonitrile solution. The mixture was stirred at room temperature for 10 minutes. Subsequently, di-tert-butyl dicarbonate (6.22 g, 28.5 mmol) was added and stirring was continued overnight at room temperature. After completion of the reaction, the reaction solution was concentrated under reduced pressure and the residue was extracted three times with dichloromethane. The organic layers were combined, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by fast column chromatography (eluent ratio methanol: dichloromethane = 2:98). Finally, the solvent was removed in vacuum to afford 4.24 g of tert-butyl (R)-(1-benzylpyrrolidin-3-yl)carbamate in 65.2% yield. The product was characterized by 1H NMR (400 MHz, CDCl3): δ 7.36-7.26 (m, 5H), 4.86 (bs, 1H), 4.18 (bs, 1H), 3.61 (s, 2H), 2.79 (bs, 1H), 2.65-2.61 (m, 1H), 2.54 (d, J=8.0 Hz, 1H), 2.34- 2.25 (m, 2H), 1.61-1.51 (m, 1H), 1.46 (s, 9H). The specific optical rotation [α]D was +2.5° (c 0.620, CHCl3).

24424-99-5
871 suppliers
$13.50/25G

114715-39-8
238 suppliers
$18.00/250mg

131878-23-4
99 suppliers
$8.00/1g
Yield: 65.2%
Reaction Conditions:
Stage #1:(R)-1-benzyl-3-aminopyrrolidine with sodium hydrogencarbonate in water;acetonitrile at 20; for 0.166667 h;
Stage #2:di-tert-butyl dicarbonate in water;acetonitrile at 20;
Steps:
1 6.2.1 Synthesis of tert-butyl (R)-(1-benzylpyrrolidin-3-yl)carbamate, 2aa
Sodium bicarbonate (5.92 g, 70.5 mmol) in 118 mL of deionized water was added to (3R)-(+)-benzylaminopyrrolidine 1a (5.00 g, 28.4 mmol) solution in 118 mL of acetonitrile and the mixture was stirred at room temperature for 10 min. Di-tert-butyl dicarbonate (6.22 g, 28.5 mmol) was then added and the mixture was stirred at room temperature overnight. After the reaction, the solution was concentrated under reduced pressure and the residue was extracted with dichloromethane three times. The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified with flash column chromatography (methanol: dichloromethane = 2:98). Removing the solvent in vacuo provided 4.24 g of tert-butyl (R)-(1-benzylpyrrolidin-3-yl)carbamate (65.2% yield). 1H NMR (400 MHz, CDCl3) δ 7.36-7.26 (m, 5H), 4.86 (bs, 1H), 4.18 (bs, 1H), 3.61 (s, 2H), 2.79 (bs, 1H), 2.65-2.61 (m, 1H), 2.54 (d, J = 8.0 Hz, 1H), 2.34-2.25 (m, 2H), 1.61-1.51 (m, 1H), 1.46 (s, 9H). [α]D +2.5° (c 0.620, CHCl3).
References:
Chough, Chieyeon;Joung, Misuk;Lee, Sunmin;Lee, Jaemin;Kim, Jong Hoon;Kim, B. Moon [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 8,p. 1495 - 1510]
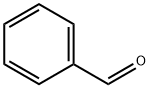
100-52-7
972 suppliers
$17.30/2g
122536-77-0
320 suppliers
$9.00/1g

131878-23-4
99 suppliers
$8.00/1g
101385-90-4
299 suppliers
$12.00/1g

131878-23-4
99 suppliers
$8.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

131878-23-4
99 suppliers
$8.00/1g
118354-71-5
19 suppliers
inquiry

131878-23-4
99 suppliers
$8.00/1g