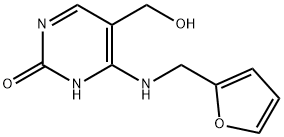
4-((furan-2-ylmethyl)amino)-5-(hydroxymethyl)pyrimidin-2(1H)-one synthesis
- Product Name:4-((furan-2-ylmethyl)amino)-5-(hydroxymethyl)pyrimidin-2(1H)-one
- CAS Number:1333466-52-6
- Molecular formula:C10H11N3O3
- Molecular Weight:221.21
Yield:1333466-52-6 95%
Reaction Conditions:
with triethylamine in water; for 8 h;Reflux;
Steps:
2
Example 2Synthesis of 4-N-aryl-5-hydroxymethylcytosineSynthesis of 4-N-aryl-5-hydroxymethylcytosine4-N-Arylcytosine, for example 4-N-furfurylcytosine (500 mg, 2.618 mmol) and paraformaldehyde (181 mg, 6.020 mmol, 2.3 eq) were placed in a flask charged with aqueous solution (10 mL) of triethylamine (3.6 mL, 0.026 mol). The entire mixture was boiled for 8 hours, and then placed in a drying oven preheated to 60°C for 24 hours. Next, the solvents were evaporated and ethanol or ethyl acetate was added to the dry residue; in this case, ethanol was used, resulting in product precipitation. After filtering the product, the filtrate was re-evaporated and precipitated (3x) with 88% yield. The reaction progress was monitored by TLC, using silica gel-covered placed and (CH2C12 : MeOH : Et3N 8:1 :0.5) or (CH2C12 : MeOH : Et3N 4:1:0.5) as eluents; in case of 4-N- furfurylcytosine, the eluent was (CH2C12 : MeOH : Et3N 4:1 :0.5). If the final product was to be an acetylated 4-N-aryl-5-hydroxymethylcytosine, the product, such as 4-N-furfuryl-5-hydroxymethylcytosine (100 mg, 0.4524 mmol) was placed in a flask containing anhydrous pyridine (0.223 mL, 2.714 mmol, not less than 5 eq relative to acetic anhydride) and acetic anhydride (0.051 mL, 0.542 mmol, 1.2 eq relative to the substrate) was added. The reaction was conducted at room temperature for 3 hours. Next, water (0.5 mL) was added to the reaction mixture and pyridine was evaporated to dryness. The product was extracted with ethyl acetate. The organic layer was evaporated to dryness and the product was subjected to freeze drying.4-N-Furfuryl-5-hydroxymethylcytosineES-MS: ES+ m/z 222 [M+H]+; 244 [M+Na]+; 465 [2M + Na]+.-NMR (400 MHz, DMSO) δ 4.1 (d, J= 4.961 Hz, 2H, H-13); 4.5 (d, J= 5.274 Hz, 2H, H-8); 5.0 (t, J= 5.166 Hz, 1H, OH); 6.2 (m, 1, H-12); 6.3 (m, 1H, H-l l); 7.2 (t, J=5.214 Hz, 1H, NH-7); 7.3 (s, 1H, H-6); 7.5 (m, 1H, H-10); 10.3 (s, 1H, N-H-l).13C- NMR (100 MHz, DMSO) δ 36.57; 57.12; 104.87; 106.87; 110.44; 140.02; 141.94; 152.26; 156.39; 163.08.
References:
WO2011/115513,2011,A2 Location in patent:Page/Page column 10-11; 16
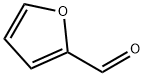
98-01-1
509 suppliers
$22.00/25g

1333466-52-6
4 suppliers
inquiry
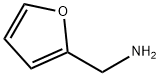
617-89-0
278 suppliers
$16.00/25g

1333466-52-6
4 suppliers
inquiry

