
Formaldehyde synthesis
- Product Name:Formaldehyde
- CAS Number:50-00-0
- Molecular formula:CH2O
- Molecular Weight:30.03
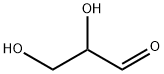
56-82-6
172 suppliers
$82.00/500mg

50-00-0
892 suppliers
$10.00/25g

64-18-6
1128 suppliers
$22.12/250ML
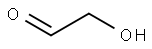
141-46-8
33 suppliers
inquiry
Yield:141-46-8 614 μmol ,64-18-6 421 μmol ,50-00-0 158 μmol
Reaction Conditions:
in water at 14.84; under 750.075 Torr; for 12 h;Sonication;Inert atmosphere;Irradiation;Electrolysis;
Steps:
2.2.1. Photoreforming experiments
General procedure: Kinetic experiments were performed in a Pyrex top-irradiationphoto-reactor connected to a closed gas-circulation system [18].The setup is equipped with facilities for online gas-analysis andliquid-phase sampling. Irradiation is provided by a 300W Xe lampwith a cold mirror 1 (CM 1). A water filter with quartz windowscloses the top of the photo-reactor. The photon flux within the reactor at water level is 8.08 x 1017 s-1 (λ < 390 nm). Typically,75 mg of photocatalyst were ultrasonically dispersed in 100 mLof a 20 mM aqueous reactant solution. The system was deaeratedby four consecutive evacuations and Ar filling cycles. All reactionswere carried out at 288 K and an Ar pressure of 1 bar.
References:
Sanwald, Kai E.;Berto, Tobias F.;Eisenreich, Wolfgang;Gutiérrez, Oliver Y.;Lercher, Johannes A. [Journal of Catalysis,2016,vol. 344,p. 806 - 816]
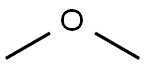
115-10-6
108 suppliers
$75.00/25g

50-00-0
892 suppliers
$10.00/25g
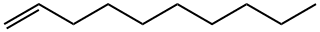
872-05-9
140 suppliers
$9.00/50g

50-00-0
892 suppliers
$10.00/25g
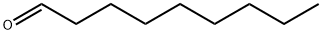
124-19-6
372 suppliers
$7.00/25g
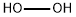
7722-84-1
0 suppliers
$24.46/*2x50ml

67-56-1
787 suppliers
$9.00/25ml

50-00-0
892 suppliers
$10.00/25g

67-56-1
787 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry

50-00-0
892 suppliers
$10.00/25g

64-17-5
826 suppliers
$10.00/50g
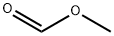
107-31-3
352 suppliers
$16.00/25mL