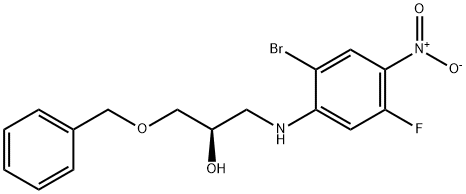
2-Propanol, 1-[(2-broMo-5-fluoro-4-nitrophenyl)aMino]-3-(phenylMethoxy)-, (2R)- synthesis
- Product Name:2-Propanol, 1-[(2-broMo-5-fluoro-4-nitrophenyl)aMino]-3-(phenylMethoxy)-, (2R)-
- CAS Number:1342896-73-4
- Molecular formula:C16H16BrFN2O4
- Molecular Weight:399.21
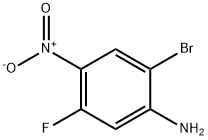
952664-69-6
136 suppliers
$7.00/250mg
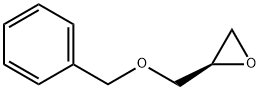
14618-80-5
275 suppliers
$5.00/1g
![2-Propanol, 1-[(2-broMo-5-fluoro-4-nitrophenyl)aMino]-3-(phenylMethoxy)-, (2R)-](/CAS/20150408/GIF/1342896-73-4.gif)
1342896-73-4
7 suppliers
inquiry
Yield:1342896-73-4 70%
Reaction Conditions:
Stage #1: 2-bromo-5-fluoro-4-nitroanilinewith zinc(II) perchlorate in toluene at 20; for 2 h;Molecular sieve;Inert atmosphere;
Stage #2: (R)-(-)-2-(benzyloxymethyl)oxirane in toluene at 85; for 15 h;Molecular sieve;Inert atmosphere;
Steps:
1.13 Step 13
Step 13[00169] (R)-l-(benzyloxy)-3-(2-bromo-5-fluoro-4-nitrophenylamino)propan-2-ol: 2- bromo-5-fluoro-4-nitroaniline (6.00 g, 25.56 mmol, 1.00 equiv.), Zn(C104)2 (1.90 g, 5.1 mmol, 0.20 equiv.), 4A Molecular Sieves (3 g), toluene (60 mL) was stirred at room temperature for 2 h and maintain with an inert atmosphere of N2 until (2R)-2- [(benzyloxy)methyl]oxirane (1.37 g, 8.34 mmol, 2.00 equiv.) was added. Then the resulting mixture was stirred for 15 h at 85 °C. The reaction progress was monitored by LCMS. The solids were filtered out and the resulting solution was diluted with 20 mL of ethyl acetate. The resulting mixture was washed with 2 x 20 mL of Sat. NH4CI and 1 x 20 mL of brine. The organic phase was dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was purified by a silica gel column, eluted with ethyl acetate/petroleum ether (1 :5) to afford 7.5 g (70%) of N-[(2R)-3-(benzyloxy)-2-hydroxypropyl]-2-bromo-5-fluoro-4- nitroaniline as a yellow solid.
References:
WO2016/109362,2016,A1 Location in patent:Paragraph 00169
2369-13-3
249 suppliers
$19.00/5g
![2-Propanol, 1-[(2-broMo-5-fluoro-4-nitrophenyl)aMino]-3-(phenylMethoxy)-, (2R)-](/CAS/20150408/GIF/1342896-73-4.gif)
1342896-73-4
7 suppliers
inquiry