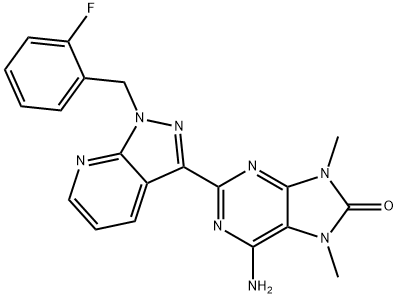
6-amino-2-(1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)- 7,9-dimethyl-7,9-dihydro-8H-purin-8-one synthesis
- Product Name:6-amino-2-(1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)- 7,9-dimethyl-7,9-dihydro-8H-purin-8-one
- CAS Number:1361569-23-4
- Molecular formula:C20H17FN8O
- Molecular Weight:404.4
![6-amino-2-(1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl) -7-methyl-7,9-dihydro-8H-purin-8-one](/CAS/20180629/GIF/1361569-18-7.gif)
1361569-18-7
36 suppliers
inquiry

74-88-4
356 suppliers
$15.00/10g
![6-amino-2-(1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)-
7,9-dimethyl-7,9-dihydro-8H-purin-8-one](/CAS/20200331/GIF/1361569-23-4.gif)
1361569-23-4
33 suppliers
inquiry
Yield:1361569-23-4 65%
Reaction Conditions:
with 2-tert-butylimino-2-diethylamino-1,3-dimethyl-perhydro-1,3,2-diazaphosphorine in N,N-dimethyl-formamide at 0; for 3 h;
Steps:
46 Example 46
6-Amino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-7,9-dimethyl-7,9-dihydro-8H-purin-8-one
Example 46
6-Amino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-7,9-dimethyl-7,9-dihydro-8H-purin-8-one
300 mg (0.768 mmol) of the compound from example 41 and 211 mg (0.768 mmol) of BEMP were initially charged in 10 ml of dimethylformamide, and a solution of 109 mg (0.768 mmol) of iodomethane in 2 ml of dimethylformamide was added dropwise at 0° C. within 10 min.
The mixture was stirred at 0° C. for 3 h.
Water was added, which formed a precipitate.
The precipitate was filtered off and purified by means of preparative HPLC (eluent: methanol/water, gradient 30:70→90:10).
203 mg of the title compound were obtained (65% of theory).
LC-MS (method 4): Rt=1.83 min; MS (EIpos): m/z=405 (M+H)+.
1H NMR (400 MHz, DMSO-d6): δ [ppm]=3.37 (s, 3H), 3.53 (s, 3H), 5.84 (s, 2H), 6.80 (s br, 2H) 7.09-7.15 (m, 2H), 7.21-7.26 (m, 1H), 7.32-7.40 (m, 2H), 8.63 (d, 1H), 9.09 (d, 1H).
References:
US2014/148433,2014,A1 Location in patent:Paragraph 1211; 1212; 1213