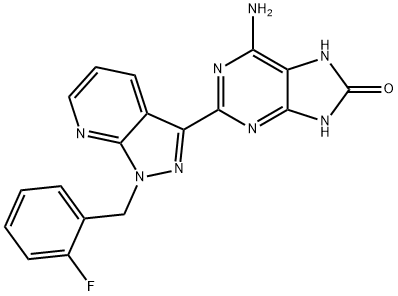
6-amino-2-(1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)- 7,9-dihydro-8H-purin-8-one synthesis
- Product Name:6-amino-2-(1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)- 7,9-dihydro-8H-purin-8-one
- CAS Number:1361569-10-9
- Molecular formula:C18H13FN8O
- Molecular Weight:376.35
530-62-1
970 suppliers
$6.00/25g
![2-[1-(2-Fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyriMidine-4,5,6-triaMine](/CAS/20150408/GIF/428854-24-4.gif)
428854-24-4
110 suppliers
$16.00/100mg
![6-amino-2-(1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)-
7,9-dihydro-8H-purin-8-one](/CAS/20200119/GIF/1361569-10-9.gif)
1361569-10-9
38 suppliers
inquiry
Yield:1361569-10-9 38%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide at 100; for 16 h;
Steps:
33 Example 33 6-Amino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-7,9-dihydro-8H-purin-8-one
Example 33
6-Amino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-7,9-dihydro-8H-purin-8-one
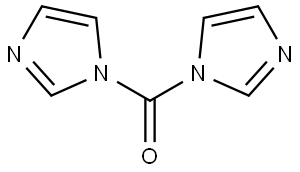
5.000 g (14.271 mmol) of the compound from example 1A were dissolved in 50 ml of dimethylformamide, and 11.570 g (71.355 mmol) of carbonyldiimidazole and 18.051 g (178.387 mmol) of triethylamine were added.
The mixture was stirred at 100° C. for 16 h.
The reaction mixture was partitioned between water and ethyl acetate, and the aqueous phase was extracted with ethyl acetate.
The combined organic phases were concentrated under reduced pressure, and the residue was stirred with tert-butyl methyl ether.
The solid formed was filtered off and purified by means of preparative HPLC (eluent: methanol/water, gradient 20:80→90:10).
2.120 g of the title compound were obtained (38% of theory).
LC-MS (method 2): Rt=0.80 min; MS (EIpos): m/z=377 (M+H)+.
1H NMR (400 MHz, DMSO-d6): δ [ppm]=5.81 (s, 2H), 6.63 (s br, 2H) 7.12-7.25 (m, 3H), 7.33-7.38 (m, 2H), 8.63 (dd, 1H), 8.96 (dd, 1H), 10.08 (s, 1H), 11.45 (s, 1H).
References:
US2014/148433,2014,A1 Location in patent:Paragraph 1160; 1161; 1162