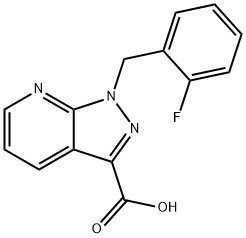
1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxylic acid synthesis
- Product Name:1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxylic acid
- CAS Number:956011-26-0
- Molecular formula:C14H10FN3O2
- Molecular Weight:271.25
![1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxylic acid ethyl ester](/CAS/20150408/GIF/256376-59-7.gif)
256376-59-7
42 suppliers
$55.00/25mg
![1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxylic acid](/CAS/20180527/GIF/956011-26-0.gif)
956011-26-0
19 suppliers
inquiry
Yield:956011-26-0 96%
Reaction Conditions:
Stage #1: ethyl 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxylatewith water;sodium hydroxide in 1,4-dioxane at 20; for 2 h;
Stage #2: with hydrogenchloride in 1,4-dioxane;water;
Steps:
29A.a 1-(2-Fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxylic acid
Step a) 1-(2-Fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxylic acid 10.0 g of ethyl 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxylate (33.41 mmol; prepared according to WO 03/095451, Example 2A) are dissolved in 500 ml of dioxane/water (1:1), and 50 ml of 1 N aqueous sodium hydroxide solution (50 mmol) are added. The reaction mixture is stirred at room temperature for 2 h and then acidified with 1 N hydrochloric acid, whereupon the product precipitates out. The product is filtered off and dried under high vacuum. This gives 8.71 g (96% of theory) of the title compound. 1H-NMR (400 MHz, DMSO-d6): δ=5.84 (s, 2H), 7.14-7.18 (m, 1H), 7.21-7.26 (m, 2H), 7.35-7.41 (m, 1H), 7.45 (dd, J=8.1, 4.6 Hz, 1H), 8.49 (dd, J=8.1, 1.5 Hz, 1H), 8.69 (dd, J=4.4, 1.2 Hz, 1H), 13.40 (br. s, 1H).
References:
US2010/4235,2010,A1 Location in patent:Page/Page column 27