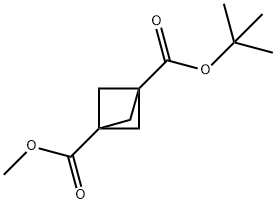
1-(tert-Butyl)3-methylbicyclo[1.1.1]pentane-1,3-dicarboxylate synthesis
- Product Name:1-(tert-Butyl)3-methylbicyclo[1.1.1]pentane-1,3-dicarboxylate
- CAS Number:138732-31-7
- Molecular formula:C12H18O4
- Molecular Weight:226.27

24424-99-5
869 suppliers
$13.50/25G
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester](/CAS/GIF/83249-10-9.gif)
83249-10-9
157 suppliers
$24.00/100mg
![1-(tert-Butyl)3-methylbicyclo[1.1.1]pentane-1,3-dicarboxylate](/CAS/GIF/138732-31-7.gif)
138732-31-7
25 suppliers
$649.00/250mg
Yield:-
Reaction Conditions:
with dmap in tert-butyl alcohol at 20; for 72 h;Inert atmosphere;
Steps:
195 5-((3-(5-(2,5-Difluoro-4-methylphenyl)-4,5-dihydro-1H-pyrazole-1-carbonyl)bicyclo[1.1.1]pentan-1-yl)methoxy)pyrazine-2-carbonitrile
1-(tert-Butyl) 3-methyl bicyclo[1.1.1]pentane-1,3-dicarboxylate To a flask containing 3-(methoxycarbonyl)bicyclo[1.1.1]pentane-1-carboxylic acid (24.7 g, 145 mmol), DMAP (5.32 g, 43.5 mmol) and Boc2O (67.4 ml, 290 mmol) was added tBuOH (97 ml), and the mixture was stirred at rt.
The reaction was vented under a stream of N2, and after the evolution of gas abated, the reaction was stirred for 3 d, by which time material solidified.
The reaction was diluted with CH2Cl2 and concentrated, then diluted with Et2O (100 ml) and washed with aqueous citric acid (250 ml, 10%), aqueous NaOH (250 ml, 0.1 M), and brine.
Each aqueous layer was extracted with the same Et2O (2*125 ml).
The organic layers were combined, dried over MgSO4, and concentrated, and residual tBuOH was azeotroped from CH2Cl2/Hexanes to give the title compound (42.7 g, 181 mmol, 125% yield) as a white waxy solid that contained 11 mole % tBuOH, and the title compound 96% pure. The material was carried on to the next step without further purification. 1H NMR (500 MHz, DMSO-d6) δ 3.61 (s, 3H), 2.19 (s, 6H), 1.40 (s, 9H).
References:
US2021/94921,2021,A1 Location in patent:Paragraph 0842-0845
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid, MonoMethyl ester](/CAS/GIF/83249-10-9.gif)
83249-10-9
157 suppliers
$24.00/100mg
![1-(tert-Butyl)3-methylbicyclo[1.1.1]pentane-1,3-dicarboxylate](/CAS/GIF/138732-31-7.gif)
138732-31-7
25 suppliers
$649.00/250mg
![Bicyclo[1.1.1]pentane-1-carboxylic acid, 3-(chlorocarbonyl)-, methyl ester](/CAS/20210111/GIF/110371-22-7.gif)
110371-22-7
4 suppliers
inquiry

75-65-0
784 suppliers
$10.00/10ml
![1-(tert-Butyl)3-methylbicyclo[1.1.1]pentane-1,3-dicarboxylate](/CAS/GIF/138732-31-7.gif)
138732-31-7
25 suppliers
$649.00/250mg