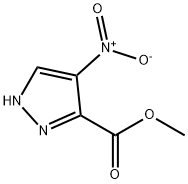
1H-Pyrazole-3-carboxylic acid, 4-nitro-, methyl ester synthesis
- Product Name:1H-Pyrazole-3-carboxylic acid, 4-nitro-, methyl ester
- CAS Number:138786-86-4
- Molecular formula:C5H5N3O4
- Molecular Weight:171.11

67-56-1
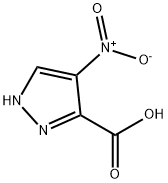
5334-40-7

138786-86-4
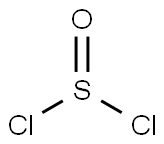
4-Nitro-1H-pyrazole-3-carboxylic acid (1.117 kg, 7.11 mol, 1.0 equiv.) and methanol (8.950 L, 8.0 v/v) were added to a 20 L reactor equipped with a digital thermometer and a mechanical stirrer. The reaction mixture was cooled to 0-5 °C under nitrogen protection and thionyl chloride (0.581 L, 7.96 mol, 0.52 v/v) was slowly added dropwise over 180 min. After the dropwise addition, the reaction mixture was gradually warmed to 18-25 °C and stirred overnight. After confirming the completion of the reaction by 1H NMR (d6-DMSO), the reaction mixture was concentrated at 40-45 °C under reduced pressure. The residue was treated with toluene (3 x 2.250 L, 3 x 2 v/v) and concentrated under reduced pressure at the same temperature to afford methyl 4-nitro-1H-pyrazole-3-carboxylate as an off-white solid (1.210 kg, 99.5% yield). In another experiment, 4-nitro-1H-pyrazole-3-carboxylic acid (1.00 kg, 6.37 mol, 1.0 eq.) and methanol (8.00 L, 8.0 v/v) were added to a flanged flask fitted with a mechanical stirrer, condenser and thermometer. The suspension was cooled to 0-5 °C under nitrogen protection and thionyl chloride (0.52 L, 7.12 mol, 0.52 v/v) was slowly added at this temperature. The mixture was gradually warmed to 15-25 °C over 16-24 h and the completion of the reaction was confirmed by 1H NMR (d6-DMSO). The reaction mixture was concentrated under reduced pressure at 35-45 °C and the residue was treated with toluene (2.00 L, 2.0 v/v) and the solvent was removed under reduced pressure at the same temperature. This process was repeated twice to give the final methyl 4-nitro-1H-pyrazole-3-carboxylate as an off-white solid (1.071 kg, 98.3% yield). In a small-scale experiment, thionyl chloride (2.90 mL, 39.8 mmol) was slowly added to a methanol (100 mL) solution of 4-nitro-1H-pyrazole-3-carboxylic acid (5.68 g, 36.2 mmol) at room temperature and the mixture was stirred for 48 hours. Upon completion of the reaction, the mixture was concentrated under reduced pressure and residual water was removed by azeotropy with toluene to afford methyl 4-nitro-1H-pyrazole-3-carboxylate as a white solid.1H NMR (400 MHz, DMSO-d6) δ 14.4 (s, 1H), 8.9 (s, 1H), 3.9 (s, 3H). Finally, 4-nitro-1H-pyrazole-3-carboxylic acid (1.350 kg, 8.59 mol, 1.0 eq.) and methanol (10.80 L, 8.0 v/v) were added to a flanged flask fitted with a mechanical stirrer, condenser and thermometer. The suspension was cooled to 0-5 °C under nitrogen protection and thionyl chloride (0.702 L, 9.62 mol, 0.52 v/v) was slowly added at this temperature. The mixture was gradually warmed to 15-25 °C over 16-24 h and the completion of the reaction was confirmed by 1H NMR (d6-DMSO). The reaction mixture was concentrated under reduced pressure at 35-45 °C and the residue was treated with toluene (2.70 L, 2.0 v/v) and the solvent was removed under reduced pressure at the same temperature. This procedure was repeated twice to give methyl 4-nitro-1H-pyrazole-3-carboxylate as an off-white solid (1.467 kg, 99.8% yield).1H NMR (d6-DMSO) was consistent with the structure and no entrapped solvent was detected.

67-56-1
790 suppliers
$7.29/5ml-f
7719-09-7
416 suppliers
$15.00/10g

5334-40-7
220 suppliers
$14.14/5gm:

138786-86-4
108 suppliers
$7.00/250mg
Yield:138786-86-4 99.5%
Reaction Conditions:
at 0 - 25; for 16 - 48 h;Product distribution / selectivity;
Steps:
4.1; 11.1
A 2OL reaction vessel equipped with a digital thermometer and stirrer was charged with A- nitro-1 H-pyrazole-3-carboxylic acid (1.117 Kg, 7.1 1 mol, 1 wt) and methanol (8.950 L, 8 vol). The reaction mixture was stirred under nitrogen, cooled to O to 5 0C, thionyl chloride (0.581 L, 8.0 mol, 0.52 vol) added over 180 minutes and the resultant mixture allowed to warm to and stir at 18 to 22 0C overnight, after which time 1H NMR analysis (d6-DMSO) indicated reaction completion. The reaction mixture was concentrated under reduced pressure at 40 to 45 0C, the residue treated with toluene and re-concentrated (3x 2.250 L, 3x 2vol) under reduced pressure at 40 to 45 0C to give 4-nitro-1H-pyrazole-3-carboxylic acid methyl ester as an off-white solid (1.210 Kg, 99.5%).; Thionyl chloride (2.90 ml, 39.8 mmol) was slowly added to a mixture of 4-nitro-3- pyrazolecarboxylic acid (5.68 g, 36.2 mmol) in MeOH (100 ml) at ambient temperature and the mixture stirred for 48 hours. The mixture was reduced in vacuo and dried through azeotrope with toluene to afford 4-nitro-1H-pyrazole-3-carboxylic acid methyl ester as a white solid.; Stage 1 : Preparation of 4-nitro-1 H-pvrazole-3-carboxylic acid methyl ester 4-Nitro-1H-pyrazole-3-carboxylic acid (1.350Kg1 8.59 MoI, 1.0 wt) and methanol (10.80L, 8.0 vol) were charged to a flange flask equipped with a mechanical stirrer, condenser and thermometer. The suspension was cooled to O to 5°C under nitrogen and thionyl chloride (0.702L, 9.62 MoI, 0.52 vol) added at this temperature. The mixture was warmed to 15 to 25°C over 16 to 24 hours. Reaction completion was determined by 1H NMR analysis (d6- DMSO). The mixture was concentrated under vacuum at 35 to 45°C and toluene (2.70L, 2.0 vol) charged to the residue and removed under vacuum at 35 to 45°C. The toluene azeotrope was repeated twice using toluene (2.70L, 2.0 vol) to give 4-nitro-1 H-pyrazole-3- carboxylic acid methyl ester [1.467Kg, 99.8%th, 108.7% w/w, 1H NMR (d6-DMSO) concordant with structure, no entrained solvent] as an off-white solid.
References:
ASTEX THERAPEUTICS LIMITED WO2008/9954, 2008, A1 Location in patent:Page/Page column 83-84; 105

5334-40-7
220 suppliers
$14.14/5gm:

138786-86-4
108 suppliers
$7.00/250mg

67-56-1
790 suppliers
$7.29/5ml-f

5334-40-7
220 suppliers
$14.14/5gm:

138786-86-4
108 suppliers
$7.00/250mg

75-36-5
588 suppliers
$17.92/100G

5334-40-7
220 suppliers
$14.14/5gm:

138786-86-4
108 suppliers
$7.00/250mg