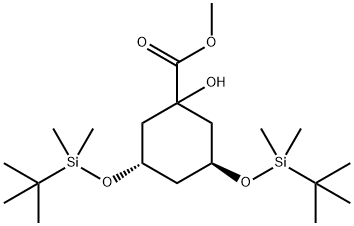
(3S,5S)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1-hydroxy-cyclohexanecarboxylic Acid Methyl Ester synthesis
- Product Name:(3S,5S)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1-hydroxy-cyclohexanecarboxylic Acid Methyl Ester
- CAS Number:139356-33-5
- Molecular formula:C20H42O5Si2
- Molecular Weight:418.72
![(1α,3R,4α,5R)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1-hydroxy-4-(1H-iMidazol-1-ylthioxoMethoxy)-cyclohexanecarboxylic Acid Methyl Ester](/CAS/GIF/139356-32-4.gif)
139356-32-4
9 suppliers
$139.80/10mg
![(3S,5S)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1-hydroxy-cyclohexanecarboxylic Acid Methyl Ester](/CAS/GIF/139356-33-5.gif)
139356-33-5
14 suppliers
$140.00/5mg
Yield:139356-33-5 100%
Reaction Conditions:
with 2,2'-azobis(isobutyronitrile);tri-n-butyl-tin hydride in toluene; for 3 h;Inert atmosphere;Reflux;
Steps:
110 Compound 3 to compound 4:
Compound 3 to compound 4: To a three necked flask was equipped with condenser and addition funnel was place a mixture of Bu3SnH (37.8g, 126mmol, 7eq) and AIBN (2.11g, 12.6mmol, 0.7eq) in toluene (200 ml). It was heated to reflux under nitrogen. The solution of compound 3 (1 .63g, 2.91 mmol) in 200mL toluene was added drop-wise via addition funnel over 1 h period. It was then refluxed for 2h. After cooled to RT, toluene was removed by Rota evaporation. The residue was purified by Flash Chromatogram on silica gel with EA/Hex. 7.5g of compound 4 was obtained (100%). Compound 4: 1H-NMR (400 MHz, CDCI3) δ 4.35 (br, 1 H), 4.25 (m, 1 H), 3.70 (s, 3H), 2.15 (m, 1 H), 1.98 (m, 1 H), 1.90 (m, 2H), 1 .65 (m, 1 H), 1 .44 (m, 1 H), 0.80 (s,s, 18H), 0.00 (s,s, 12H).
References:
WO2013/90929,2013,A1 Location in patent:Page/Page column 124; 125
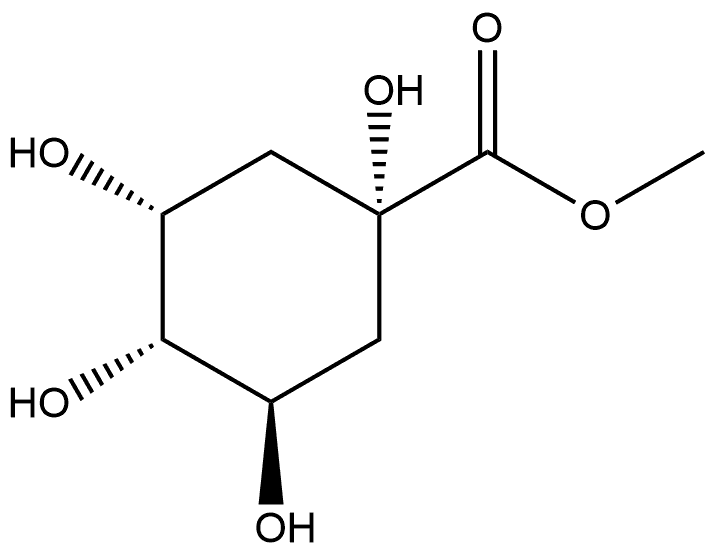
191916-39-9
0 suppliers
inquiry
![(3S,5S)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1-hydroxy-cyclohexanecarboxylic Acid Methyl Ester](/CAS/GIF/139356-33-5.gif)
139356-33-5
14 suppliers
$140.00/5mg
![(1α,3R,4α,5R)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1,4-dihydroxy-cyclohexanecarboxylic Acid Methyl Ester](/CAS/GIF/135711-62-5.gif)
135711-62-5
11 suppliers
$150.00/25mg
![(3S,5S)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1-hydroxy-cyclohexanecarboxylic Acid Methyl Ester](/CAS/GIF/139356-33-5.gif)
139356-33-5
14 suppliers
$140.00/5mg
77-95-2
316 suppliers
$19.80/5G
![(3S,5S)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1-hydroxy-cyclohexanecarboxylic Acid Methyl Ester](/CAS/GIF/139356-33-5.gif)
139356-33-5
14 suppliers
$140.00/5mg
138-59-0
632 suppliers
$5.00/250mg
![(3S,5S)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1-hydroxy-cyclohexanecarboxylic Acid Methyl Ester](/CAS/GIF/139356-33-5.gif)
139356-33-5
14 suppliers
$140.00/5mg