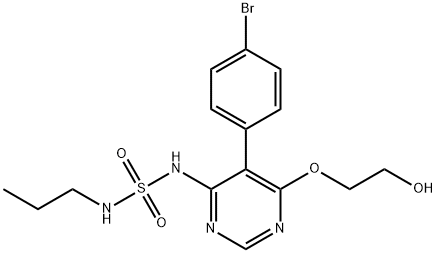
SulfaMide, N-[5-(4-broMophenyl)-6-(2-hydroxyethoxy)-4-pyriMidinyl]-N'-propyl- synthesis
- Product Name:SulfaMide, N-[5-(4-broMophenyl)-6-(2-hydroxyethoxy)-4-pyriMidinyl]-N'-propyl-
- CAS Number:1393813-43-8
- Molecular formula:C15H19BrN4O4S
- Molecular Weight:431.3
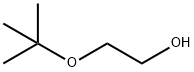
7580-85-0
277 suppliers
$32.00/25mL
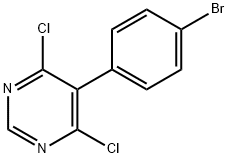
![SulfaMide, N-[5-(4-broMophenyl)-6-chloro-4-pyriMidinyl]-N'-propyl-](/CAS/20150408/GIF/1393813-42-7.gif)
1393813-42-7
114 suppliers
inquiry
![SulfaMide, N-[5-(4-broMophenyl)-6-(2-hydroxyethoxy)-4-pyriMidinyl]-N'-propyl-](/CAS/20150408/GIF/1393813-43-8.gif)
1393813-43-8
105 suppliers
inquiry
Yield:1393813-43-8 86%
Reaction Conditions:
Stage #1: N-5-(4-bromophenyl)-6-chloro-4-pyrimidinyl-N‘-propylsulfamidewith potassium tert-butylate in toluene at 20; for 0.0833333 h;
Stage #2: tert-butyl glycidyl ether in toluene at 85; for 2 h;
Stage #3: with titanium tetrachloride in toluene at 35; for 20 h;
Steps:
3 Example 3: N-(5-(4-bromophenyl)-6-(2-hydroxyethoxy)pyrimidin-4-yl)propane- 1-sulfamide (one pot preparation):
Example 3: N-(5-(4-bromophenyl)-6-(2-hydroxyethoxy)pyrimidin-4-yl)propane- 1-sulfamide (one pot preparation):N-(5-(4-bromophenyl)-6-chloropyrimidin-4-yl)propane-1-sulfamide (100 g; 0.246 mol; see Preparation A) and KOtBu (110 g; 0.99 mol; 4.0 eq.) were charged into a 2 L reactor. Toluene (1 L) was added. The resulting suspension was stirred at 20°C for 5 mm. 2-(tert-butoxy)ethanol (97 mL; 0.74 mol; 3.0 eq.) was added dropwise. After completion of the addition, the reaction mixture was heated to 85°C for 2 h. After completion of the reaction, the mixture was cooled down to 20°C. Water (0.5 L) was added, followed by the addition of 10% aq. citric acid (0.5 L). The layers were separated. The org. phase was washed with brine 3 times (0.5 L each) and azeotropically dried with toluene (1 L) to a volume of approx. 1 L. The reaction mixture was heated to 50°C. A 1M solution of TiC14 in toluene (420 mL, 0.42 mol; 1.7 eq.) was added dropwise with vigorous stirring. After completion of the addition, it was stirred at 35°C for 20 h. Water (0.75 L) was added and the resulting beige suspension was stirred for 15 h. It was filtered off, rinsed with toluene (300 mL) and dried to afford the title compound as a white solid (91 g; 86% yield; purity (LC-MS): 100% a/a).The product had NIVIR data equivalent to those reported in Bolli et al., J. Med. Chem. (2012), 55, 7849-7861.
References:
WO2014/155304,2014,A1 Location in patent:Page/Page column 27; 28
![SulfaMide, N-[5-(4-broMophenyl)-6-chloro-4-pyriMidinyl]-N'-propyl-](/CAS/20150408/GIF/1393813-42-7.gif)
1393813-42-7
114 suppliers
inquiry
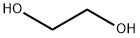
107-21-1
1380 suppliers
$10.00/25g
![SulfaMide, N-[5-(4-broMophenyl)-6-(2-hydroxyethoxy)-4-pyriMidinyl]-N'-propyl-](/CAS/20150408/GIF/1393813-43-8.gif)
1393813-43-8
105 suppliers
inquiry
146533-41-7
315 suppliers
$16.00/1g
![SulfaMide, N-[5-(4-broMophenyl)-6-(2-hydroxyethoxy)-4-pyriMidinyl]-N'-propyl-](/CAS/20150408/GIF/1393813-43-8.gif)
1393813-43-8
105 suppliers
inquiry
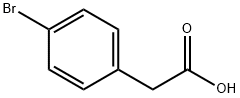
1878-68-8
509 suppliers
$6.00/10g
![SulfaMide, N-[5-(4-broMophenyl)-6-(2-hydroxyethoxy)-4-pyriMidinyl]-N'-propyl-](/CAS/20150408/GIF/1393813-43-8.gif)
1393813-43-8
105 suppliers
inquiry
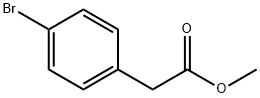
41841-16-1
216 suppliers
$5.00/5g
![SulfaMide, N-[5-(4-broMophenyl)-6-(2-hydroxyethoxy)-4-pyriMidinyl]-N'-propyl-](/CAS/20150408/GIF/1393813-43-8.gif)
1393813-43-8
105 suppliers
inquiry