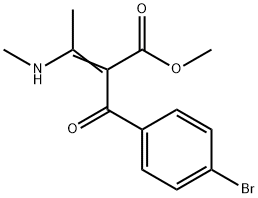
Benzenepropanoic acid, 4-bromo-α-[1-(methylamino)ethylidene]-β-oxo-, methyl ester synthesis
- Product Name:Benzenepropanoic acid, 4-bromo-α-[1-(methylamino)ethylidene]-β-oxo-, methyl ester
- CAS Number:1423699-80-2
- Molecular formula:C13H14BrNO3
- Molecular Weight:312.1591
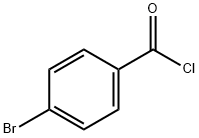
586-75-4
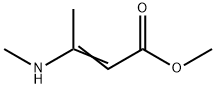
13412-12-9
![Benzenepropanoic acid, 4-bromo-α-[1-(methylamino)ethylidene]-β-oxo-, methyl ester](/CAS/20180713/GIF/1423699-80-2.gif)
1423699-80-2
To a stirred solution of tetrahydrofuran (THF) containing methyl 3-(methylamino)but-2-enoate (1.00 g, 7.82 mmol) and pyridine (0.74 mL, 7.82 mmol) was added slowly and dropwise 4-bromobenzoyl chloride (1.71 g, 7.82 mmol) at 0 °C and protected by nitrogen. After the dropwise addition, the reaction was continued with stirring at 0 °C for 0.5 h, followed by slow warming to room temperature and stirring overnight. Upon completion of the reaction, the reaction was quenched by the addition of water (60 mL) and extracted with ethyl acetate (60 mL x 3). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 10:1) to afford the target product methyl 2-(4-bromobenzoyl)-3-(methylamino)but-2-enoate (0.8 g, 33% yield).
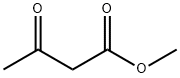
105-45-3
694 suppliers
$5.00/5g
![Benzenepropanoic acid, 4-bromo-α-[1-(methylamino)ethylidene]-β-oxo-, methyl ester](/CAS/20180713/GIF/1423699-80-2.gif)
1423699-80-2
14 suppliers
$28.00/100mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: methanol / 18 h / 45 °C
2: pyridine / tetrahydrofuran / 0 - 20 °C / Inert atmosphere
References:
WO2013/25733,2013,A1
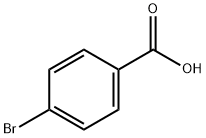
586-76-5
505 suppliers
$10.00/1g
![Benzenepropanoic acid, 4-bromo-α-[1-(methylamino)ethylidene]-β-oxo-, methyl ester](/CAS/20180713/GIF/1423699-80-2.gif)
1423699-80-2
14 suppliers
$28.00/100mg