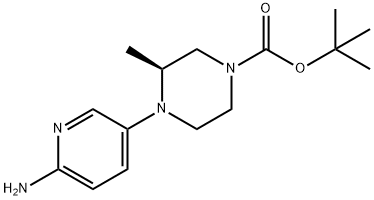
tert-butyl(S)-4-(6-aminopyridin-3-yl)-3-methylpiperazine-1-carboxylate synthesis
- Product Name:tert-butyl(S)-4-(6-aminopyridin-3-yl)-3-methylpiperazine-1-carboxylate
- CAS Number:1433849-58-1
- Molecular formula:C15H24N4O2
- Molecular Weight:292.38
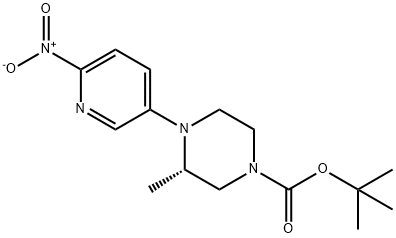
1433849-53-6
10 suppliers
inquiry

1433849-58-1
20 suppliers
inquiry
Yield:1433849-58-1 96%
Reaction Conditions:
with Pd/C;hydrogen in ethanol at 20; for 16 h;Inert atmosphere;
Steps:
105.b (3S)-tert-Butyl 4-(6-Aminopyridin-3-yl)-3-methylpiperazine-1-carboxylate 105b
Example 105b
(3S)-tert-Butyl 4-(6-Aminopyridin-3-yl)-3-methylpiperazine-1-carboxylate 105b
A 500-mL bottle was purged with nitrogen and charged with 105a (5.8 g, 18 mmol), 10% palladium on carbon (50% wet, 2.0 g) and ethanol (200 mL).
It was evacuated, charged with hydrogen gas, and stirred for 16 h at room temperature.
The hydrogen was then removed in vacuo and replaced with nitrogen.
The catalyst was removed by filtration through a pad of CELITE and the filtrate concentrated under reduced pressure to afford 105b as a brown solid (4.9 g, 96%). LCMS: [M+H]+ 293
References:
US2013/116246,2013,A1 Location in patent:Paragraph 0255; 0256
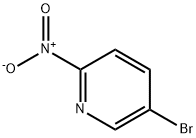
39856-50-3
546 suppliers
$6.00/5g

1433849-58-1
20 suppliers
inquiry
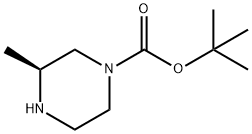
147081-29-6
333 suppliers
$5.00/1g

1433849-58-1
20 suppliers
inquiry
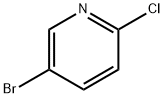
53939-30-3
482 suppliers
$5.00/1g

1433849-58-1
20 suppliers
inquiry