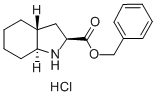
Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride synthesis
- Product Name:Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride
- CAS Number:145641-35-6
- Molecular formula:C16H22ClNO2
- Molecular Weight:295.8
144540-75-0
64 suppliers
inquiry

100-51-6
1434 suppliers
$5.00/100g

145641-35-6
36 suppliers
inquiry
Yield:145641-35-6 90.1%
Reaction Conditions:
with thionyl chloride in dichloromethane;
Steps:
19
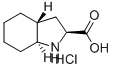
Example 19(26',3ai?,7a5)-Octahvdro-lH-indole-2-carboxylic acid hydrochloride (5); (5)In a 500 mL reaction flask equipped with a distillation head was added 33.5 g (0.143 mol, 1.0 equiv.) of (25f,3ai?,7a5)-Ethyl octahydro-lH-indole-2-carboxylate hydrochloride (16), 102 g of water, and 102 g of cone, hydrochloric acid. The contents were heated to 94-96 0C temperature with mixing for NLT 6 h while collecting about 9 mL of distillate at atmospheric pressure. The reaction mixture was cooled to room temperature and concentrated to dryness on a rotary evaporator. To the resulting crude product, 255 mL of acetonitrile was added and heated for 1 h at reflux with mixing to break up and dissolve the solids. The mixture was cooled gradually to 0-5 0C temperature at a rate of 10-15 0C per h, and held at 0-5 0C for NLT 2 h under nitrogen atmosphere. The solids were collected via filtration and washed with 10-20 mL of chilled (0-5 0C) acetonitrile. The product was dried at 45 0C under vacuum for 16 h to afford 25.6 g of (25',3ai?,7a5)-Octahydro-lH-indole-2-carboxylic acid hydrochloride (5) in 86.9% yield as a white solid. 1H NMR (D2O, 400 MHz): δ 4.42 (dd, 1 H, J= 11.1, 2.7 Hz), 2.93, (dt, 1 H, J = 11.8, 3.6 Hz), 2.36 (ddd, 1 H, J= 12.9, 6.7, 2.7 Hz), 2.31 - 2.16 (m, 1 H), 2.11 - 2.01 (m, 2 H), 1.92 - 1.90 (m, 1 H), 1.79 - 1.75 (m, 1 H), 1.68 - 1.53 (m, 2 H), 1.34 - 1.13 (m, 3 H); LC-MS (m/z): 170.1 (M+H)+. The isolated product (5) correlates to the material prepared according to US487932 and Tetrahedron Lett., 1992, 33, 4889. Further, ^S^aiJa^-Octahydro-lH-mdole^-carboxylic acid hydrochloride (5, 25.0 g, 0.122 mol) is converted to the corresponding benzyl ester [(25*,3ai?,7a5)-Benzyl octahydro-lH- indole-2-carboxylate hydrochloride] using thionyl chloride, benzyl alcohol in dichloromethane in 90.1% yield (32.7 g) and the product was correlated by ΗPLC and NMR to the to the material prepared according to US487932 and Tetrahedron Lett. 1992, 33, 4889. Analytical ΗPLC (Daicel Chiralpack AD-RΗ column, Size: 150 x 4.6 mm); 0.02 M Ammonium acetate buffer (pΗ: 7.7-7.8):Acetonitrile/50:50, 0.3 mL/min, 220 nm, Column chamber temperature: 55 0C, R^: 32.6 min, 99.34 % (PA); 1H NMR (D2O, 400 MHz): δ 7.49 - 7.43 (m, 5 H), 5.37 - 5.28 (q, 2 H, J= 23.2, 12.0 Hz), 4.60 (dd, 1 H, J= 11.2, 2.9 Hz), 2.95, (dt, 1 H, J= 11.8, 3.7 Hz), 2.36 (ddd, 1 H, J= 13.1, 6.9, 2.9 Hz), 2.25 - 2.15 (m, 1 H), 2.13 - 2.02 (m, 2 H), 2.03 - 1.96 (m, 1 H), 1.95 - 1.88 (m, 1 H), 1.65 - 1.53 (m, 2 H), 1.32 - 1.12 (m, 3 H); LC-MS (m/z): 274.1 (M+H)+.
References:
WO2011/9021,2011,A1 Location in patent:Page/Page column 39-40
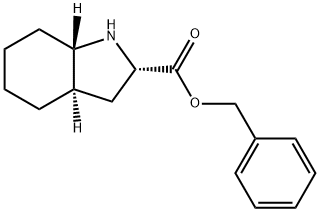
144540-71-6
13 suppliers
inquiry

145641-35-6
36 suppliers
inquiry
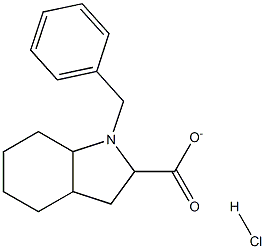
82717-97-3
7 suppliers
inquiry

145641-35-6
36 suppliers
inquiry
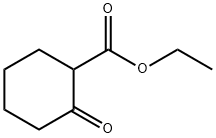
1655-07-8
308 suppliers
$6.00/5g

145641-35-6
36 suppliers
inquiry
![1-Cyclohexene-1-carboxylic acid, 2-[[(1S)-1-phenylethyl]amino]-, ethyl ester](/CAS/20210305/GIF/53898-03-6.gif)
53898-03-6
0 suppliers
inquiry

145641-35-6
36 suppliers
inquiry