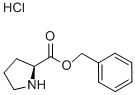
L-Proline benzyl ester hydrochloride synthesis
- Product Name:L-Proline benzyl ester hydrochloride
- CAS Number:16652-71-4
- Molecular formula:C12H16ClNO2
- Molecular Weight:241.71
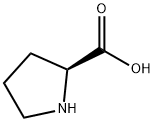
147-85-3

100-51-6

16652-71-4
Under nitrogen protection, benzyl alcohol (70 mL, 651 mmol) was cooled to 0 °C and thionyl chloride (7.0 mL, 91.2 mmol) was added slowly and dropwise. L-proline (5.0 g, 43.4 mmol) was then added, and the reaction system was kept stirred at 0 °C and under nitrogen atmosphere for 2 hours. The temperature was slowly increased to room temperature and stirring was continued for 48 hours. Upon completion of the reaction, the reaction mixture was slowly poured into ether (300 mL) and left to crystallize at -20°C for 7 days. The resulting white solid precipitate was collected by filtration, washed with ether and dried under vacuum to give L-proline benzyl ester hydrochloride (9.88 g, 93% yield). The product characterization data were as follows: melting point 142.1-144.0 °C (literature value: 143-144 °C); 1H NMR (300 MHz, CDCl3): δ 7.41-7.21 (m, 5H), 5.16 (s, 2H), 3.80 (dd, J = 3.83, 5.9 Hz, 1H), 3.15-3.01 (m, 1H), 3.00-2.82 (m, 1H), 2.42-2.21 (m, 1H), 2.13 (dd, J = 12.9, 7.5 Hz, 1H), 1.92-1.62 (m, 3H); 13C NMR (75 MHz, CDCl3): δ 175.5, 136.0, 128.8, 128.5, 128.3, 66.9, 59.9, 47.2, 30.4, 25.6. Elemental analysis (C12H16ClNO2) calculated values: C, 59.63; H, 6.67; N, 5.79; measured values: C, 59.50; H, 6.86; N, 5.64.

147-85-3
1060 suppliers
$6.00/25g

100-51-6
1434 suppliers
$5.00/100g

16652-71-4
337 suppliers
$6.00/5g
Yield:16652-71-4 93%
Reaction Conditions:
Stage #1:benzyl alcohol with thionyl chloride at 0;Inert atmosphere;
Stage #2:L-proline at 0 - 20; for 50 h;Inert atmosphere;
Steps:
2.4 (S)-2-((Benzyloxy)carbonyl)pyrrolidin-1-ium chloride (8)
Benzyl alcohol (70 mL, 651 mmol) was cooled to 0 °C under nitrogen and 7.0 mL thionyl chloride (91.2 mmol) was added. l-Proline (5.0 g, 43.4 mmol) was then added and the mixture was stirred at 0 °C under nitrogen for 2 h.
The mixture was warmed to room temperature and stirring continued for 48 h.
The reaction mixture was then poured into 300 mL diethyl ether and stored at -20 °C for 7 days.
The precipitate formed was collected by filtration, washed with diethyl ether, and dried under vacuum to give 8 as white solid (9.88 g, 93% yield); mp 142.1-144.0 °C; lit: 143-144 °C. 1H NMR (300 MHz, CDCl3): δ 7.41-7.21 (m, 5H), 5.16 (s, 2H), 3.80 (dd, J = 3.83, 5.9 Hz, 1H), 3.15-3.01 (m, 1H), 3.00-2.82 (m, 1H), 2.42-2.21 (m, 1H), 2.13 (dd, J = 12.9 Hz, 7.5 Hz, 1H), 1.92-1.62 (m, 3H).
13C NMR (75 MHz, CDCl3): δ 175.5, 136.0, 128.8, 128.5, 128.3, 66.9, 59.9, 47.2, 30.4, 25.6. Anal. Calcd for C12H16ClNO2: C, 59.63; H, 6.67; N, 5.79. Found: C, 59.50; H, 6.86; N, 5.64.
25
References:
Li, Zhiliang;Lebedyeva, Iryna O.;Golubovskaya, Vita M.;Cance, William G.;Alamry, Khalid A.;Faidallah, Hassan M.;Dennis Hall;Katritzky, Alan R. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 15,p. 5056 - 5060]
37787-77-2
0 suppliers
inquiry

16652-71-4
337 suppliers
$6.00/5g

100-51-6
1434 suppliers
$5.00/100g

16652-71-4
337 suppliers
$6.00/5g
15761-39-4
608 suppliers
$5.00/10g

16652-71-4
337 suppliers
$6.00/5g